Liver Tropism in Cancer: The Hepatic Metastatic Niche
- PMID: 31548227
- PMCID: PMC7050581
- DOI: 10.1101/cshperspect.a037259
Liver Tropism in Cancer: The Hepatic Metastatic Niche
Abstract
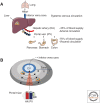
The liver is the largest organ in the human body and is prone for cancer metastasis. Although the metastatic pattern can differ depending on the cancer type, the liver is the organ to which cancer cells most frequently metastasize for the majority of prevalent malignancies. The liver is unique in several aspects: the vascular structure is highly permeable and has unparalleled dual blood connectivity, and the hepatic tissue microenvironment presents a natural soil for the seeding of disseminated tumor cells. Although 70% of the liver is composed of the parenchymal hepatocytes, the remaining 30% is composed of nonparenchymal cells including Kupffer cells, liver sinusoidal endothelial cells, and hepatic stellate cells. Recent discoveries show that both the parenchymal and the nonparenchymal cells can modulate each step of the hepatic metastatic cascade, including the initial seeding and colonization as well as the decision to undergo dormancy versus outgrowth. Thus, a better understanding of the molecular mechanisms orchestrating the formation of a hospitable hepatic metastatic niche and the identification of the drivers supporting this process is critical for the development of better therapies to stop or at least decrease liver metastasis. The focus of this perspective is on the bidirectional interactions between the disseminated cancer cells and the unique hepatic metastatic niche.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Aychek T, Miller K, Sagi-Assif O, Levy-Nissenbaum O, Israeli-Amit M, Pasmanik-Chor M, Jacob-Hirsch J, Amariglio N, Rechavi G, Witz IP. 2008. E-selectin regulates gene expression in metastatic colorectal carcinoma cells and enhances HMGB1 release. Int J Cancer 123: 1741–1750. 10.1002/ijc.23375 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
