"It Takes a Village": Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms
- PMID: 31548277
- PMCID: PMC6932244
- DOI: 10.1128/JB.00530-19
"It Takes a Village": Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms
Abstract
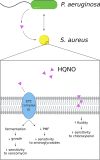
Chronic infections are frequently caused by polymicrobial biofilms. Importantly, these infections are often difficult to treat effectively in part due to the recalcitrance of biofilms to antimicrobial therapy. Emerging evidence suggests that polymicrobial interactions can lead to dramatic and unexpected changes in the ability of antibiotics to eradicate biofilms and often result in decreased antimicrobial efficacy in vitro In this review, we discuss the influence of polymicrobial interactions on the antibiotic susceptibility of biofilms, and we highlight the studies that first documented the shifted antimicrobial susceptibilities of mixed-species cultures. Recent studies have identified several mechanisms underlying the recalcitrance of polymicrobial biofilm communities, including interspecies exchange of antibiotic resistance genes, β-lactamase-mediated inactivation of antibiotics, changes in gene expression induced by metabolites and quorum sensing signals, inhibition of the electron transport chain, and changes in properties of the cell membrane. In addition to elucidating multiple mechanisms that contribute to the altered drug susceptibility of polymicrobial biofilms, these studies have uncovered novel ways in which polymicrobial interactions can impact microbial physiology. The diversity of findings discussed highlights the importance of continuing to investigate the efficacy of antibiotics against biofilm communities composed of different combinations of microbial species. Together, the data presented here illustrate the importance of studying microbes as part of mixed-species communities rather than in isolation. In light of our greater understanding of how interspecies interactions alter the efficacy of antimicrobial agents, we propose that the methods for measuring the drug susceptibility of polymicrobial infections should be revisited.
Keywords: antibiotics; biofilm; polymicrobial; recalcitrance; resistance; tolerance.
Copyright © 2019 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

