Clonogenic assay of gastric adenocarcinoma stem cells--clonogenic assay, stomach cancer
- PMID: 3154829
- PMCID: PMC4534928
- DOI: 10.3904/kjim.1987.2.2.163
Clonogenic assay of gastric adenocarcinoma stem cells--clonogenic assay, stomach cancer
Abstract
Clonogenic assay is the culturing of tumor stem cells, which are responsible for tumor growth by self renewal and differentiation to tumor end cells. Elimination of the malignant stem cells or their self renewal will lead cure of the tumor. Thus, the behavior of the stem cells by clonogenic assay has been correlated with prognosis and outcome of therapy.
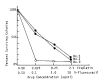
We studied clonogenic assay by means of double agar system for 21 patients with advanced stomach cancer. The colony formation evaluted on 14th day of the culture was grown from 14 of 19 malignant effusions plated and 1 of 2 tumor nodules plated. The number of the colonies ranged from 5 to 96 per petri dish, and the median number was 20. The plating efficiency ranged from 0.001 to 0.036%, and the median was 0.003%. In the morphologic studies, the colonies made of tightly packed cells were grown from the poorly differentiated adenocarcinoma, whereas the colonies made of loosely packed cells with mucin formation were grown from the mucin secreting adenocarcinoma of stomach. The chemosensitivity in vitro tests to cisplatin were performed in 5 patients, and the results showed 4 sensitive and 1 resistant patient. The result of in vivo study with instillation of intraperitoneal cisplatin revealed that 3 patients “in vitro sensitive” were “responsive in vivo”, and 1 patient “in vitro resistant” was “non-responsive in vivo”.
We conclude that clonogenic assay of stomach cancer is useful method to understand the biology of the stem cell pool and select proper chemotherapy according to the chemosensitivity test.
Figures

Similar articles
-
Significance of surgical adjuvant chemotherapy for gastric cancer.J Surg Oncol. 1991 Mar;46(3):203-7. doi: 10.1002/jso.2930460316. J Surg Oncol. 1991. PMID: 2011033
-
A Rare Case Report of Skin Metastasis in Gastric Cancer.J Gastrointest Cancer. 2021 Sep;52(3):1156-1158. doi: 10.1007/s12029-021-00603-3. Epub 2021 Feb 26. J Gastrointest Cancer. 2021. PMID: 33635503 No abstract available.
-
Emerging Novel Therapeutic Agents in the Treatment of Patients with Gastroesophageal and Gastric Adenocarcinoma.Hematol Oncol Clin North Am. 2017 Jun;31(3):529-544. doi: 10.1016/j.hoc.2017.02.001. Epub 2017 Mar 22. Hematol Oncol Clin North Am. 2017. PMID: 28501092 Review.
-
Gastric cancer stem cells: a novel therapeutic target.Cancer Lett. 2013 Sep 10;338(1):110-9. doi: 10.1016/j.canlet.2013.03.035. Epub 2013 Apr 10. Cancer Lett. 2013. PMID: 23583679 Free PMC article. Review.
-
NANOGP8 is the key regulator of stemness, EMT, Wnt pathway, chemoresistance, and other malignant phenotypes in gastric cancer cells.PLoS One. 2018 Apr 24;13(4):e0192436. doi: 10.1371/journal.pone.0192436. eCollection 2018. PLoS One. 2018. PMID: 29689047 Free PMC article.
Cited by
-
Chemosensitivity and resistance testing in malignant effusions with focus on primary malignant mesothelioma and metastatic adenocarcinoma.Pleura Peritoneum. 2016 Sep 1;1(3):119-133. doi: 10.1515/pp-2016-0013. Epub 2016 Sep 15. Pleura Peritoneum. 2016. PMID: 30911616 Free PMC article. Review.
-
EGFL6 promotes colorectal cancer cell growth and mobility and the anti-cancer property of anti-EGFL6 antibody.Cell Biosci. 2021 Mar 16;11(1):53. doi: 10.1186/s13578-021-00561-0. Cell Biosci. 2021. PMID: 33726836 Free PMC article.
-
β-Catenin Regulates the Expression of cAMP Response Element-Binding Protein 1 in Squamous Cell Carcinoma Cells.Ann Dermatol. 2018 Feb;30(1):119-122. doi: 10.5021/ad.2018.30.1.119. Epub 2017 Dec 26. Ann Dermatol. 2018. PMID: 29386853 Free PMC article. No abstract available.
References
-
- Ministry of Health and Social Affairs Three years’ report for cancer register programme in Republic of Korea (July 1, 1980∼June 30, 1983) J Kor Cancer Res Assoc. 1984;16:73.
-
- MacDonald JS, Woolley PV, Smythe T, Ueno SV, Hoth D, Shein PS. 5-Fluorouracil, adriamycin, and mitomycin-C (FAM) combination chemotherapy in the treatment of advanced gastric cancer. Cancer. 1979;44:42. - PubMed
-
- Moon H, Kim HK, Kim CC, Yoo JY, Lee KS, Park DH, Kim BS, Kim DJ. The efficacy of High-FAM in advanced gastric cancer. J Kor Cancer Res Assoc. 1984;16:272.
-
- Von Hoff DD, Clark GM, Stogdil BJ. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983;43:749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
