Transbronchial Invasion and Proliferation of Leptospira interrogans in Lung without Inflammatory Cell Infiltration in a Hamster Model
- PMID: 31548321
- PMCID: PMC6867870
- DOI: 10.1128/IAI.00727-19
Transbronchial Invasion and Proliferation of Leptospira interrogans in Lung without Inflammatory Cell Infiltration in a Hamster Model
Abstract
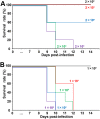
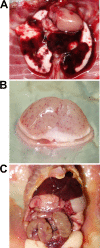
Leptospirosis caused by pathogenic Leptospira is one of the most common zoonoses in the world. It is believed that humans become infected with it mainly through their skin and mucous membranes by contact with water or soil that is contaminated with urine excreted from infected animals. Recently, outbreaks have frequently occurred in the tropics, especially after flooding, but how leptospires cause mass infection remains poorly understood. In this study, we injected leptospires into the tracheas of hamsters under direct view and prove for the first time that leptospires can infect through the respiratory tract. We determined that a 50% lethal dose (LD50) of the Leptospira interrogans strain UP-MMC-SM (L495) for hamsters in transtracheal infection was 3.2 × 102 cells. The results of culture, macroscopic findings, and histopathological analysis suggested that intratracheally injected leptospires invaded the lung tissue, proliferated in the collagen-rich stroma adjacent to the bronchus and blood vessels, and then spread throughout the body via the bloodstream. In the lung, leptospires continuously infiltrated the alveolar wall without inflammatory cell infiltration, spread throughout the lung, and finally caused pulmonary hemorrhage. Our results revealed that the respiratory tract might be a portal of entry for leptospires. We speculate that some cases of leptospirosis might be caused by transbronchial infection from inhaling infectious aerosols containing leptospires during floods. Leptospira was also confirmed to be a unique pathogen that invades through the bronchus, proliferates in the collagen-rich lung stroma, and spreads through the alveolar interstitium throughout the lung without causing pneumonia.
Keywords: Leptospira interrogans; aerosols; bronchoalveolar lavage fluid; collagen; hamster; leptospirosis; lung; pulmonary hemorrhage; respiratory tract infection; transbronchial infection.
Copyright © 2019 American Society for Microbiology.
Figures




Similar articles
-
Correlation between renal distribution of leptospires during the acute phase and chronic renal dysfunction in a hamster model of infection with Leptospira interrogans.PLoS Negl Trop Dis. 2021 Jun 18;15(6):e0009410. doi: 10.1371/journal.pntd.0009410. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34143778 Free PMC article.
-
Dissemination of Leptospira into the intestinal tract resulting in fecal excretion in a hamster model of subcutaneous infection with Leptospirainterrogans.Microb Pathog. 2022 Apr;165:105481. doi: 10.1016/j.micpath.2022.105481. Epub 2022 Mar 12. Microb Pathog. 2022. PMID: 35292370
-
Adipose tissue is the first colonization site of Leptospira interrogans in subcutaneously infected hamsters.PLoS One. 2017 Feb 28;12(2):e0172973. doi: 10.1371/journal.pone.0172973. eCollection 2017. PLoS One. 2017. PMID: 28245231 Free PMC article.
-
Pathology and pathophysiology of pulmonary manifestations in leptospirosis.Braz J Infect Dis. 2007 Feb;11(1):142-8. doi: 10.1590/s1413-86702007000100029. Braz J Infect Dis. 2007. PMID: 17625743 Review.
-
Leptospirosis is an invasive infectious and systemic inflammatory disease.Biomed J. 2020 Feb;43(1):24-31. doi: 10.1016/j.bj.2019.12.002. Epub 2020 Feb 21. Biomed J. 2020. PMID: 32200953 Free PMC article. Review.
Cited by
-
Degradation of p0071 and p120-catenin during adherens junction disassembly by Leptospira interrogans.Front Cell Infect Microbiol. 2023 Sep 15;13:1228051. doi: 10.3389/fcimb.2023.1228051. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37795382 Free PMC article.
-
Correlation between renal distribution of leptospires during the acute phase and chronic renal dysfunction in a hamster model of infection with Leptospira interrogans.PLoS Negl Trop Dis. 2021 Jun 18;15(6):e0009410. doi: 10.1371/journal.pntd.0009410. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34143778 Free PMC article.
-
Disassembly of the apical junctional complex during the transmigration of Leptospira interrogans across polarized renal proximal tubule epithelial cells.Cell Microbiol. 2021 Sep;23(9):e13343. doi: 10.1111/cmi.13343. Epub 2021 May 6. Cell Microbiol. 2021. PMID: 33864347 Free PMC article.
-
Non-purulent myositis caused by direct invasion of skeletal muscle tissue by Leptospira in a hamster model.Infect Immun. 2024 Feb 13;92(2):e0042023. doi: 10.1128/iai.00420-23. Epub 2024 Jan 19. Infect Immun. 2024. PMID: 38240601 Free PMC article.
-
Pulmonary haemorrhage as the earliest sign of severe leptospirosis in hamster model challenged with Leptospira interrogans strain HP358.PLoS Negl Trop Dis. 2022 May 18;16(5):e0010409. doi: 10.1371/journal.pntd.0010409. eCollection 2022 May. PLoS Negl Trop Dis. 2022. PMID: 35584087 Free PMC article.
References
-
- World Health Organization and International Leptospirosis Society. 2003. Human leptospirosis: guidance for diagnosis, surveillance and control. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/handle/10665/42667.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

