The Acinetobacter baumannii Znu System Overcomes Host-Imposed Nutrient Zinc Limitation
- PMID: 31548324
- PMCID: PMC6867866
- DOI: 10.1128/IAI.00746-19
The Acinetobacter baumannii Znu System Overcomes Host-Imposed Nutrient Zinc Limitation
Abstract
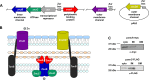
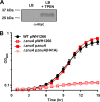
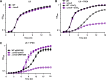
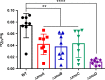
Acinetobacter baumannii is an opportunistic bacterial pathogen capable of causing a variety of infections, including pneumonia, sepsis, wound, and burn infections. A. baumannii is an increasing threat to public health due to the prevalence of multidrug-resistant strains, leading the World Health Organization to declare A. baumannii a "Priority 1: Critical" pathogen, for which the development of novel antimicrobials is desperately needed. Zinc (Zn) is an essential nutrient that pathogenic bacteria, including A. baumannii, must acquire from their hosts in order to survive. Consequently, vertebrate hosts have defense mechanisms to sequester Zn from invading bacteria through a process known as nutritional immunity. Here, we describe a
Keywords: Acinetobacter; metal transporters; zinc.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration.PLoS Pathog. 2012;8(12):e1003068. doi: 10.1371/journal.ppat.1003068. Epub 2012 Dec 6. PLoS Pathog. 2012. PMID: 23236280 Free PMC article.
-
An Acinetobacter baumannii, Zinc-Regulated Peptidase Maintains Cell Wall Integrity during Immune-Mediated Nutrient Sequestration.Cell Rep. 2019 Feb 19;26(8):2009-2018.e6. doi: 10.1016/j.celrep.2019.01.089. Cell Rep. 2019. PMID: 30784584 Free PMC article.
-
The Response of Acinetobacter baumannii to Zinc Starvation.Cell Host Microbe. 2016 Jun 8;19(6):826-36. doi: 10.1016/j.chom.2016.05.007. Cell Host Microbe. 2016. PMID: 27281572 Free PMC article.
-
The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host.Front Cell Infect Microbiol. 2013 Dec 12;3:95. doi: 10.3389/fcimb.2013.00095. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24377089 Free PMC article. Review.
-
Tug of war between Acinetobacter baumannii and host immune responses.Pathog Dis. 2018 Dec 1;76(9):ftz004. doi: 10.1093/femspd/ftz004. Pathog Dis. 2018. PMID: 30657912 Review.
Cited by
-
The zinc metalloprotein MigC impacts cell wall biogenesis through interactions with an essential Mur ligase in Acinetobacter baumannii.PLoS Pathog. 2025 Jun 16;21(6):e1013209. doi: 10.1371/journal.ppat.1013209. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40523033 Free PMC article.
-
Conformational flexibility in the zinc solute-binding protein ZnuA.Acta Crystallogr F Struct Biol Commun. 2022 Mar 1;78(Pt 3):128-134. doi: 10.1107/S2053230X22001662. Epub 2022 Feb 28. Acta Crystallogr F Struct Biol Commun. 2022. PMID: 35234138 Free PMC article.
-
Antimicrobial peptides: Defending the mucosal epithelial barrier.Front Oral Health. 2022 Aug 1;3:958480. doi: 10.3389/froh.2022.958480. eCollection 2022. Front Oral Health. 2022. PMID: 35979535 Free PMC article. Review.
-
Acinetobacter Metabolism in Infection and Antimicrobial Resistance.Infect Immun. 2023 Jun 15;91(6):e0043322. doi: 10.1128/iai.00433-22. Epub 2023 May 16. Infect Immun. 2023. PMID: 37191522 Free PMC article. Review.
-
Development and Evaluation of an Immunoinformatics-Based Multi-Peptide Vaccine against Acinetobacter baumannii Infection.Vaccines (Basel). 2024 Mar 27;12(4):358. doi: 10.3390/vaccines12040358. Vaccines (Basel). 2024. PMID: 38675740 Free PMC article.
References
-
- Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats. Centers for Disease Control and Prevention, Atlanta, GA.
-
- Tacconelli E, Magrini N. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

