Rhinovirus-Induced SIRT-1 via TLR2 Regulates Subsequent Type I and Type III IFN Responses in Airway Epithelial Cells
- PMID: 31548332
- PMCID: PMC6810856
- DOI: 10.4049/jimmunol.1900165
Rhinovirus-Induced SIRT-1 via TLR2 Regulates Subsequent Type I and Type III IFN Responses in Airway Epithelial Cells
Abstract
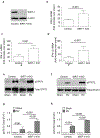
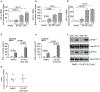
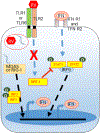
IFN responses to viral infection are necessary to establish intrinsic antiviral state, but if unchecked can lead to heightened inflammation. Recently, we showed that TLR2 activation contributes to limitation of rhinovirus (RV)-induced IFN response in the airway epithelial cells. We also demonstrated that compared with normal airway epithelial cells, those from patients with chronic obstructive pulmonary disease (COPD) show higher IFN responses to RV, but the underlying mechanisms are not known. Initially, RV-induced IFN responses depend on dsRNA receptor activation and then are amplified via IFN-stimulated activation of JAK/STAT signaling. In this study, we show that in normal cells, TLR2 limits RV-induced IFN responses by attenuating STAT1 and STAT2 phosphorylation and this was associated with TLR2-dependent SIRT-1 expression. Further, inhibition of SIRT-1 enhanced RV-induced IFN responses, and this was accompanied by increased STAT1/STAT2 phosphorylation, indicating that TLR2 may limit RV-induced IFN responses via SIRT-1. COPD airway epithelial cells showed attenuated IL-8 responses to TLR2 agonist despite expressing TLR2 similar to normal, indicating dysregulation in TLR2 signaling pathway. Unlike normal, COPD cells failed to show RV-induced TLR2-dependent SIRT-1 expression. Pretreatment with quercetin, which increases SIRT-1 expression, normalized RV-induced IFN levels in COPD airway epithelial cells. Inhibition of SIRT-1 in quercetin-pretreated COPD cells abolished the normalizing effects of quercetin on RV-induced IFN expression in these cells, confirming that quercetin exerts its effect via SIRT-1. In summary, we show that TLR2 is required for limiting RV-induced IFNs, and this pathway is dysregulated in COPD airway epithelial cells, leading to exaggerated IFN production.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures



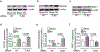



References
-
- Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, Schoonbroodt S, Tuck AC, Kim V, Ostridge K, Staples KJ, Williams N, Williams A, Wootton S, Devaster JM, and Group AS. 2017. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 72: 919–927. - PMC - PubMed
-
- Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, and Johnston SL. 2011. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183: 734–742. - PMC - PubMed
-
- Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL, Martinez FJ, Hershenson MB, and Sajjan U. 2010. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 332–340. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

