Kidney Regeneration in Later-Stage Mouse Embryos via Transplanted Renal Progenitor Cells
- PMID: 31548350
- PMCID: PMC6900792
- DOI: 10.1681/ASN.2019020148
Kidney Regeneration in Later-Stage Mouse Embryos via Transplanted Renal Progenitor Cells
Abstract
Background: The limited availability of donor kidneys for transplantation has spurred interest in investigating alternative strategies, such as regenerating organs from stem cells transplanted into animal embryos. However, there is no known method for transplanting cells into later-stage embryos, which may be the most suitable host stages for organogenesis, particularly into regions useful for kidney regeneration.
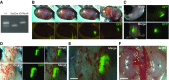
Methods: We demonstrated accurate transplantation of renal progenitor cells expressing green fluorescent protein to the fetal kidney development area by incising the opaque uterine muscle layer but not the transparent amniotic membrane. We allowed renal progenitor cell-transplanted fetuses to develop for 6 days postoperatively before removal for analysis. We also transplanted renal progenitor cells into conditional kidney-deficient mouse embryos. We determined growth and differentiation of transplanted cells in all cases.
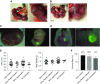
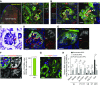
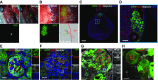
Results: Renal progenitor cell transplantation into the retroperitoneal cavity of fetuses at E13-E14 produced transplant-derived, vascularized glomeruli with filtration function and did not affect fetal growth or survival. Cells transplanted to the nephrogenic zone produced a chimera in the cap mesenchyme of donor and host nephron progenitor cells. Renal progenitor cells transplanted to conditional kidney-deficient fetuses induced the formation of a new nephron in the fetus that is connected to the host ureteric bud.
Conclusions: We developed a cell transplantation method for midstage to late-stage fetuses. In vivo kidney regeneration from renal progenitor cells using the renal developmental environment of the fetus shows promise. Our findings suggest that fetal transplantation methods may contribute to organ regeneration and developmental research.
Keywords: kidney development; kidney regeneration; nephron; pluripotent stem cell; progenitor; stem cell.
Copyright © 2019 by the American Society of Nephrology.
Figures





Comment in
-
Prioritizing Functional Goals as We Rebuild the Kidney.J Am Soc Nephrol. 2019 Dec;30(12):2287-2288. doi: 10.1681/ASN.2019101051. Epub 2019 Nov 1. J Am Soc Nephrol. 2019. PMID: 31676726 Free PMC article. No abstract available.
References
-
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. .: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 - PubMed
-
- Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, et al. .: Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol 17: 1026–1034, 2006 - PubMed
-
- Wu J, Greely HT, Jaenisch R, Nakauchi H, Rossant J, Belmonte JC: Stem cells and interspecies chimaeras. Nature 540: 51–59, 2016 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

