Parkinson's disease-associated iPLA2-VIA/ PLA2G6 regulates neuronal functions and α-synuclein stability through membrane remodeling
- PMID: 31548400
- PMCID: PMC6789907
- DOI: 10.1073/pnas.1902958116
Parkinson's disease-associated iPLA2-VIA/ PLA2G6 regulates neuronal functions and α-synuclein stability through membrane remodeling
Abstract
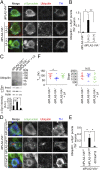
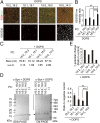
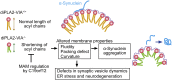
Mutations in the iPLA2-VIA/PLA2G6 gene are responsible for PARK14-linked Parkinson's disease (PD) with α-synucleinopathy. However, it is unclear how iPLA2-VIA mutations lead to α-synuclein (α-Syn) aggregation and dopaminergic (DA) neurodegeneration. Here, we report that iPLA2-VIA-deficient Drosophila exhibits defects in neurotransmission during early developmental stages and progressive cell loss throughout the brain, including degeneration of the DA neurons. Lipid analysis of brain tissues reveals that the acyl-chain length of phospholipids is shortened by iPLA2-VIA loss, which causes endoplasmic reticulum (ER) stress through membrane lipid disequilibrium. The introduction of wild-type human iPLA2-VIA or the mitochondria-ER contact site-resident protein C19orf12 in iPLA2-VIA-deficient flies rescues the phenotypes associated with altered lipid composition, ER stress, and DA neurodegeneration, whereas the introduction of a disease-associated missense mutant, iPLA2-VIA A80T, fails to suppress these phenotypes. The acceleration of α-Syn aggregation by iPLA2-VIA loss is suppressed by the administration of linoleic acid, correcting the brain lipid composition. Our findings suggest that membrane remodeling by iPLA2-VIA is required for the survival of DA neurons and α-Syn stability.
Keywords: Drosophila; ER stress; Parkinson’s disease; lipids; α-synuclein.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Phospholipase PLA2G6, a Parkinsonism-Associated Gene, Affects Vps26 and Vps35, Retromer Function, and Ceramide Levels, Similar to α-Synuclein Gain.Cell Metab. 2018 Oct 2;28(4):605-618.e6. doi: 10.1016/j.cmet.2018.05.019. Epub 2018 Jun 14. Cell Metab. 2018. PMID: 29909971
-
Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction.Brain. 2015 Jul;138(Pt 7):1801-16. doi: 10.1093/brain/awv132. Epub 2015 May 22. Brain. 2015. PMID: 26001724 Free PMC article.
-
PARK14 (D331Y) PLA2G6 Causes Early-Onset Degeneration of Substantia Nigra Dopaminergic Neurons by Inducing Mitochondrial Dysfunction, ER Stress, Mitophagy Impairment and Transcriptional Dysregulation in a Knockin Mouse Model.Mol Neurobiol. 2019 Jun;56(6):3835-3853. doi: 10.1007/s12035-018-1118-5. Epub 2018 Aug 8. Mol Neurobiol. 2019. PMID: 30088174
-
The Overcrowded Crossroads: Mitochondria, Alpha-Synuclein, and the Endo-Lysosomal System Interaction in Parkinson's Disease.Int J Mol Sci. 2019 Oct 25;20(21):5312. doi: 10.3390/ijms20215312. Int J Mol Sci. 2019. PMID: 31731450 Free PMC article. Review.
-
Parkinson's Disease-Related Genes and Lipid Alteration.Int J Mol Sci. 2021 Jul 16;22(14):7630. doi: 10.3390/ijms22147630. Int J Mol Sci. 2021. PMID: 34299248 Free PMC article. Review.
Cited by
-
Serum metabolomic characterization of PLA2G6-associated dystonia-parkinsonism: A case-control biomarker study.Front Neurosci. 2022 Aug 11;16:879548. doi: 10.3389/fnins.2022.879548. eCollection 2022. Front Neurosci. 2022. PMID: 36033628 Free PMC article.
-
Emerging Disease-Modifying Therapies in Neurodegeneration With Brain Iron Accumulation (NBIA) Disorders.Front Neurol. 2021 Apr 15;12:629414. doi: 10.3389/fneur.2021.629414. eCollection 2021. Front Neurol. 2021. PMID: 33935938 Free PMC article. Review.
-
Light-driven activation of mitochondrial proton-motive force improves motor behaviors in a Drosophila model of Parkinson's disease.Commun Biol. 2019 Nov 22;2:424. doi: 10.1038/s42003-019-0674-1. eCollection 2019. Commun Biol. 2019. PMID: 31799427 Free PMC article.
-
Dynamic Role of Phospholipases A2 in Health and Diseases in the Central Nervous System.Cells. 2021 Oct 30;10(11):2963. doi: 10.3390/cells10112963. Cells. 2021. PMID: 34831185 Free PMC article. Review.
-
Lipids: Key Players That Modulate α-Synuclein Toxicity and Neurodegeneration in Parkinson's Disease.Int J Mol Sci. 2020 May 7;21(9):3301. doi: 10.3390/ijms21093301. Int J Mol Sci. 2020. PMID: 32392751 Free PMC article. Review.
References
-
- Kalia L. V., Lang A. E., Parkinson’s disease. Lancet 386, 896–912 (2015). - PubMed
-
- Auluck P. K., Caraveo G., Lindquist S., α-Synuclein: Membrane interactions and toxicity in Parkinson’s disease. Annu. Rev. Cell Dev. Biol. 26, 211–233 (2010). - PubMed
-
- Bozek K., et al. , Organization and evolution of brain lipidome revealed by large-scale analysis of human, chimpanzee, macaque, and mouse tissues. Neuron 85, 695–702 (2015). - PubMed
-
- Antonny B., Vanni S., Shindou H., Ferreira T., From zero to six double bonds: Phospholipid unsaturation and organelle function. Trends Cell Biol. 25, 427–436 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

