Individual and collective encoding of risk in animal groups
- PMID: 31548427
- PMCID: PMC6789631
- DOI: 10.1073/pnas.1905585116
Individual and collective encoding of risk in animal groups
Abstract
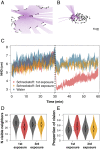
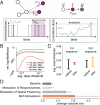
The need to make fast decisions under risky and uncertain conditions is a widespread problem in the natural world. While there has been extensive work on how individual organisms dynamically modify their behavior to respond appropriately to changing environmental conditions (and how this is encoded in the brain), we know remarkably little about the corresponding aspects of collective information processing in animal groups. For example, many groups appear to show increased "sensitivity" in the presence of perceived threat, as evidenced by the increased frequency and magnitude of repeated cascading waves of behavioral change often observed in fish schools and bird flocks under such circumstances. How such context-dependent changes in collective sensitivity are mediated, however, is unknown. Here we address this question using schooling fish as a model system, focusing on 2 nonexclusive hypotheses: 1) that changes in collective responsiveness result from changes in how individuals respond to social cues (i.e., changes to the properties of the "nodes" in the social network), and 2) that they result from changes made to the structural connectivity of the network itself (i.e., the computation is encoded in the "edges" of the network). We find that despite the fact that perceived risk increases the probability for individuals to initiate an alarm, the context-dependent change in collective sensitivity predominantly results not from changes in how individuals respond to social cues, but instead from how individuals modify the spatial structure, and correspondingly the topology of the network of interactions, within the group. Risk is thus encoded as a collective property, emphasizing that in group-living species individual fitness can depend strongly on coupling between scales of behavioral organization.
Keywords: antipredator behavior; group structure; social contagion.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lima S., Dill L., Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 68, 619–640 (1990).
-
- Sih A., Optimal behavior: Can foragers balance two conflicting demands? Science 210, 1041–1043 (1980). - PubMed
-
- Milinski M., “Predation risk and feeding behaviour” in Behaviour of Teleost Fishes, Pitcher T., Ed. (Chapman and Hall, London, ed. 2, 1993), pp. 285–305.
-
- Lima S., Bednekoff P., Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 153, 649–659 (1999). - PubMed
-
- Pitcher T., Parrish J., “Functions of shoaling behaviour in Teleosts ” in Behaviour of Teleost Fishes, Pitcher T., Ed. (Chapman and Hall, London, ed. 2, 1993), pp. 363–439.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

