Antibodies and Derivatives Targeting DR4 and DR5 for Cancer Therapy
- PMID: 31548531
- PMCID: PMC6698863
- DOI: 10.3390/antib6040016
Antibodies and Derivatives Targeting DR4 and DR5 for Cancer Therapy
Abstract
Developing therapeutics that induce apoptosis in cancer cells has become an increasingly attractive approach for the past 30 years. The discovery of tumor necrosis factor (TNF) superfamily members and more specifically TNF-related apoptosis-inducing ligand (TRAIL), the only cytokine of the family capable of eradicating selectively cancer cells, led to the development of numerous TRAIL derivatives targeting death receptor 4 (DR4) and death receptor 5 (DR5) for cancer therapy. With a few exceptions, preliminary attempts to use recombinant TRAIL, agonistic antibodies, or derivatives to target TRAIL agonist receptors in the clinic have been fairly disappointing. Nonetheless, a tremendous effort, worldwide, is being put into the development of novel strategic options to target TRAIL receptors. Antibodies and derivatives allow for the design of novel and efficient agonists. We summarize and discuss here the advantages and drawbacks of the soar of TRAIL therapeutics, from the first developments to the next generation of agonistic products, with a particular insight on new concepts.
Keywords: TRAIL; antibody; antibody drug conjugate; apoptosis; bi-specific; cancer therapy; chimeric antigen receptor; death-receptor targeting; ligand; scFv.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



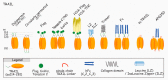
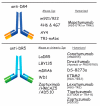
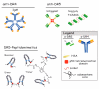

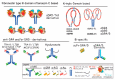

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

