The emerging role of epigenetic therapeutics in immuno-oncology
- PMID: 31548600
- PMCID: PMC7254932
- DOI: 10.1038/s41571-019-0266-5
The emerging role of epigenetic therapeutics in immuno-oncology
Abstract
The past decade has seen the emergence of immunotherapy as a prime approach to cancer treatment, revolutionizing the management of many types of cancer. Despite the promise of immunotherapy, most patients do not have a response or become resistant to treatment. Thus, identifying combinations that potentiate current immunotherapeutic approaches will be crucial. The combination of immune-checkpoint inhibition with epigenetic therapy is one such strategy that is being tested in clinical trials, encompassing a variety of cancer types. Studies have revealed key roles of epigenetic processes in regulating immune cell function and mediating antitumour immunity. These interactions make combined epigenetic therapy and immunotherapy an attractive approach to circumvent the limitations of immunotherapy alone. In this Review, we highlight the basic dynamic mechanisms underlying the synergy between immunotherapy and epigenetic therapies and detail current efforts to translate this knowledge into clinical benefit for patients.
Conflict of interest statement
Competing interests
S.B.B. is an inventor of the methylation-specific PCR platform, which is licensed to MDxHealth in agreement with Johns Hopkins University; S.B.B. and Johns Hopkins University are entitled to royalty sales shares. S.B.B. is on the Scientific Advisory Board for Mirati Therapeutics. J.R.B. is on advisory board/consultant for Amgen, BMS (uncompensated), Celgene, Genentech, Janssen Oncology, Lilly, Merck and Syndax. J.R.B. recieves grant research funding from AstraZeneca/MedImmune, BMS and Merck. K.A.M. is a consultant for AstraZeneca. All other authors declare no competing interests.
Figures

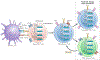
References
-
- Robert C et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med 364, 2517–2526 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

