Active PLK1-driven metastasis is amplified by TGF-β signaling that forms a positive feedback loop in non-small cell lung cancer
- PMID: 31548612
- PMCID: PMC6976524
- DOI: 10.1038/s41388-019-1023-z
Active PLK1-driven metastasis is amplified by TGF-β signaling that forms a positive feedback loop in non-small cell lung cancer
Erratum in
-
Correction: Active PLK1-driven metastasis is amplified by TGF-β signaling that forms a positive feedback loop in non-small cell lung cancer.Oncogene. 2020 Jan;39(4):951. doi: 10.1038/s41388-019-1049-2. Oncogene. 2020. PMID: 31595031 Free PMC article.
Abstract
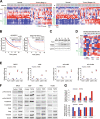
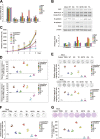
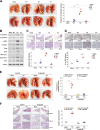
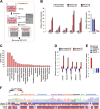
Early findings that PLK1 is highly expressed in cancer have driven an exploration of its functions in metastasis. However, whether PLK1 induces metastasis in vivo and its underlying mechanisms in NSCLC have not yet been determined. Here, we show that the expression of active PLK1 phosphorylated at T210, abundant in TGF-β-treated lung cells, potently induced metastasis in a tail-vein injection model. Active PLK1 with intact polo-box and ATP-binding domains accelerated cell motility and invasiveness by triggering EMT reprogramming, whereas a phosphomimetic version of p-S137-PLK1 did not, indicating that the phosphorylation status of PLK1 may determine the cell traits. Active PLK1-driven invasiveness upregulated TGF-β signaling and TSG6 encoded by TNFAIP6. Loss of TNFAIP6 disturbed the metastatic activity induced by active PLK1 or TGF-β. Clinical relevance shows that PLK1 and TNFAIP6 are strong predictors of poor survival rates in metastatic NSCLC patients. Therefore, we suggest that active PLK1 promotes metastasis by upregulating TGF-β signaling, which amplifies its metastatic properties by forming a positive feedback loop and that the PLK1/TGF-β-driven metastasis is effectively blocked by targeting PLK1 and TSG6, providing PLK1 and TSG6 as negative markers for prognostics and therapeutic targets in metastatic NSCLC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
PLK1-mediated phosphorylation of β-catenin enhances its stability and transcriptional activity for extracellular matrix remodeling in metastatic NSCLC.Theranostics. 2023 Feb 5;13(3):1198-1216. doi: 10.7150/thno.79318. eCollection 2023. Theranostics. 2023. PMID: 36793862 Free PMC article.
-
MicroRNA-100 is a potential molecular marker of non-small cell lung cancer and functions as a tumor suppressor by targeting polo-like kinase 1.BMC Cancer. 2012 Nov 14;12:519. doi: 10.1186/1471-2407-12-519. BMC Cancer. 2012. PMID: 23151088 Free PMC article.
-
Epithelial-Mesenchymal Transition Predicts Polo-Like Kinase 1 Inhibitor-Mediated Apoptosis in Non-Small Cell Lung Cancer.Clin Cancer Res. 2016 Apr 1;22(7):1674-1686. doi: 10.1158/1078-0432.CCR-14-2890. Epub 2015 Nov 23. Clin Cancer Res. 2016. PMID: 26597303 Free PMC article.
-
Non-canonical cMet regulation by vimentin mediates Plk1 inhibitor-induced apoptosis.EMBO Mol Med. 2019 May;11(5):e9960. doi: 10.15252/emmm.201809960. EMBO Mol Med. 2019. PMID: 31040125 Free PMC article.
-
BIX02189 inhibits TGF-β1-induced lung cancer cell metastasis by directly targeting TGF-β type I receptor.Cancer Lett. 2016 Oct 28;381(2):314-22. doi: 10.1016/j.canlet.2016.08.010. Epub 2016 Aug 16. Cancer Lett. 2016. PMID: 27543359
Cited by
-
Identification of potential circular RNA biomarkers in lung adenocarcinoma: A bioinformatics analysis and retrospective clinical study.Oncol Lett. 2022 May;23(5):144. doi: 10.3892/ol.2022.13264. Epub 2022 Mar 11. Oncol Lett. 2022. PMID: 35340554 Free PMC article.
-
An immune gene signature to predict prognosis and immunotherapeutic response in lung adenocarcinoma.Sci Rep. 2022 May 17;12(1):8230. doi: 10.1038/s41598-022-12301-6. Sci Rep. 2022. PMID: 35581376 Free PMC article.
-
Comprehensive Analyses of Ferroptosis-Related Alterations and Their Prognostic Significance in Glioblastoma.Front Mol Biosci. 2022 Jun 3;9:904098. doi: 10.3389/fmolb.2022.904098. eCollection 2022. Front Mol Biosci. 2022. PMID: 35720126 Free PMC article.
-
PLK1 regulates hepatic stellate cell activation and liver fibrosis through Wnt/β-catenin signalling pathway.J Cell Mol Med. 2020 Jul;24(13):7405-7416. doi: 10.1111/jcmm.15356. Epub 2020 May 28. J Cell Mol Med. 2020. PMID: 32463161 Free PMC article.
-
Analysis of the Expression of Cell Division Cycle-Associated Genes and Its Prognostic Significance in Human Lung Carcinoma: A Review of the Literature Databases.Biomed Res Int. 2020 Feb 12;2020:6412593. doi: 10.1155/2020/6412593. eCollection 2020. Biomed Res Int. 2020. PMID: 32104702 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

