CA1-projecting subiculum neurons facilitate object-place learning
- PMID: 31548723
- PMCID: PMC6819262
- DOI: 10.1038/s41593-019-0496-y
CA1-projecting subiculum neurons facilitate object-place learning
Abstract
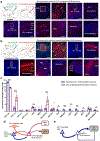
Recent anatomical evidence suggests a functionally significant back-projection pathway from the subiculum to the CA1. Here we show that the afferent circuitry of CA1-projecting subicular neurons is biased by inputs from CA1 inhibitory neurons and the visual cortex, but lacks input from the entorhinal cortex. Efferents of the CA1-projecting subiculum neurons also target the perirhinal cortex, an area strongly implicated in object-place learning. We identify a critical role for CA1-projecting subicular neurons in object-location learning and memory, and show that this projection modulates place-specific activity of CA1 neurons and their responses to displaced objects. Together, these experiments reveal a novel pathway by which cortical inputs, particularly those from the visual cortex, reach the hippocampal output region CA1. Our findings also implicate this circuitry in the formation of complex spatial representations and learning of object-place associations.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures


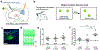

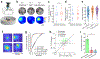
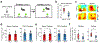
Similar articles
-
Noncanonical projections to the hippocampal CA3 regulate spatial learning and memory by modulating the feedforward hippocampal trisynaptic pathway.PLoS Biol. 2021 Dec 20;19(12):e3001127. doi: 10.1371/journal.pbio.3001127. eCollection 2021 Dec. PLoS Biol. 2021. PMID: 34928938 Free PMC article.
-
Responses of rat subicular neurons to convergent stimulation of lateral entorhinal cortex and CA1 in vivo.Brain Res. 2000 Nov 24;884(1--2):35-50. doi: 10.1016/s0006-8993(00)02878-x. Brain Res. 2000. PMID: 11082485
-
Plastic and metaplastic changes in the CA1 and subicular projections to the entorhinal cortex.Brain Res. 2007 May 25;1147:124-39. doi: 10.1016/j.brainres.2007.02.016. Epub 2007 Feb 21. Brain Res. 2007. PMID: 17368431
-
[Involvement of trisynaptic hippocampal pathway in generation of neural representations of object-place associations (analytical review)].Zh Vyssh Nerv Deiat Im I P Pavlova. 2009 Nov-Dec;59(6):643-59. Zh Vyssh Nerv Deiat Im I P Pavlova. 2009. PMID: 20120726 Review. Russian.
-
Noncanonical connections between the subiculum and hippocampal CA1.J Comp Neurol. 2016 Dec 1;524(17):3666-3673. doi: 10.1002/cne.24024. Epub 2016 May 6. J Comp Neurol. 2016. PMID: 27150503 Free PMC article. Review.
Cited by
-
New rabies viral resources for multi-scale neural circuit mapping.Mol Psychiatry. 2024 Jul;29(7):1951-1967. doi: 10.1038/s41380-024-02451-6. Epub 2024 Feb 14. Mol Psychiatry. 2024. PMID: 38355784 Free PMC article.
-
Longitudinal dynamics of microvascular recovery after acquired cortical injury.Acta Neuropathol Commun. 2022 Apr 25;10(1):59. doi: 10.1186/s40478-022-01361-4. Acta Neuropathol Commun. 2022. PMID: 35468870 Free PMC article.
-
HippoBellum: Acute Cerebellar Modulation Alters Hippocampal Dynamics and Function.J Neurosci. 2020 Sep 2;40(36):6910-6926. doi: 10.1523/JNEUROSCI.0763-20.2020. Epub 2020 Aug 7. J Neurosci. 2020. PMID: 32769107 Free PMC article.
-
Cognitively impaired aged Octodon degus recapitulate major neuropathological features of sporadic Alzheimer's disease.Acta Neuropathol Commun. 2022 Dec 19;10(1):182. doi: 10.1186/s40478-022-01481-x. Acta Neuropathol Commun. 2022. PMID: 36529803 Free PMC article.
-
Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer's disease.Nat Commun. 2022 Feb 22;13(1):998. doi: 10.1038/s41467-022-28493-4. Nat Commun. 2022. PMID: 35194025 Free PMC article.
References
Methods-only references
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

