Fertility Preservation in Women With Malignancy: Future Endeavors
- PMID: 31548799
- PMCID: PMC6743198
- DOI: 10.1177/1179558119872490
Fertility Preservation in Women With Malignancy: Future Endeavors
Abstract
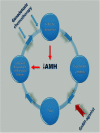
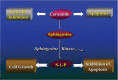
The area of fertility preservation is constantly developing. To date, the only noninvestigational and unequivocally accepted methods for fertility preservation are cryopreservation of embryos and unfertilized oocytes. This article is one of several in a monogram on fertility preservation. The debate, pros and cons, and equivocal data on the use of GnRH analogues for fertility preservation are elaborated by 3 other manuscripts, in this monogram. A repeat of the arguments, pros and cons of this debatable issue, would be a repetition and redundancy of what is already included in this monogram. The subject of ovarian cryopreservation for fertility preservation is also elaborated by several other authors in this monogram. It is possible that, in the not too far future, the technologies of in vitro maturation of primordial follicles to metaphase 2 oocytes, and the "artificial ovary," will turn clinically available. These technologies may bypass the risk of resuming malignancy by autotransplantation of cryopreserved-thawed ovarian tissue in leukemia and diseases where malignant cells may persist in the cryopreserved ovarian tissue. We summarize here the suggested options for future endeavors in fertility preservation.
Keywords: Fertility preservation; GnRH analogues; chemotherapy; premature ovarian failure (POF); premature ovarian insufficiency (POI).
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Preservation of fertility and ovarian function and minimalization of chemotherapy associated gonadotoxicity and premature ovarian failure: the role of inhibin-A and -B as markers.Mol Cell Endocrinol. 2002 Feb 22;187(1-2):93-105. doi: 10.1016/s0303-7207(01)00712-2. Mol Cell Endocrinol. 2002. PMID: 11988316 Review.
-
Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy.Hum Reprod. 2016 Apr;31(4):750-62. doi: 10.1093/humrep/dew007. Epub 2016 Feb 4. Hum Reprod. 2016. PMID: 26848188
-
Fertility after treatment for Hodgkin's disease.Ann Oncol. 2002;13 Suppl 1:138-47. doi: 10.1093/annonc/13.s1.138. Ann Oncol. 2002. PMID: 12078896 Review.
-
Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women.J Soc Gynecol Investig. 1999 Sep-Oct;6(5):229-39. doi: 10.1016/s1071-5576(99)00028-3. J Soc Gynecol Investig. 1999. PMID: 10554760 Review.
-
Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy.Clin Med Insights Reprod Health. 2019 Aug 21;13:1179558119870163. doi: 10.1177/1179558119870163. eCollection 2019. Clin Med Insights Reprod Health. 2019. PMID: 31488958 Free PMC article. Review.
Cited by
-
Exosomes from adipose-derived stem cells alleviate premature ovarian failure via blockage of autophagy and AMPK/mTOR pathway.PeerJ. 2023 Dec 14;11:e16517. doi: 10.7717/peerj.16517. eCollection 2023. PeerJ. 2023. PMID: 38107591 Free PMC article.
-
Human adipose-derived stromal cells transplantation prolongs reproductive lifespan on mouse models of mild and severe premature ovarian insufficiency.Stem Cell Res Ther. 2021 Oct 10;12(1):537. doi: 10.1186/s13287-021-02590-5. Stem Cell Res Ther. 2021. PMID: 34629095 Free PMC article.
-
Breast cancer Treatment and Fertility Preservation: A Narrative Review of Impacts, Strategies and Ethical Considerations.Curr Oncol Rep. 2024 Dec;26(12):1575-1585. doi: 10.1007/s11912-024-01619-1. Epub 2024 Nov 27. Curr Oncol Rep. 2024. PMID: 39602057 Review.
-
Encapsulated Allografts Preclude Host Sensitization and Promote Ovarian Endocrine Function in Ovariectomized Young Rhesus Monkeys and Sensitized Mice.Bioengineering (Basel). 2023 May 3;10(5):550. doi: 10.3390/bioengineering10050550. Bioengineering (Basel). 2023. PMID: 37237620 Free PMC article.
References
-
- Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008; 14:543-552. - PubMed
-
- Meirow D, Assad G, Dor J, Rabinovici J. The GnRH antagonist cetrorelix reduces cyclophosphamide-induced ovarian follicular destruction in mice. Hum Reprod. 2004;19:1294-1299. - PubMed
-
- Danforth DR, Arbogast LK, Friedman CI. Acute depletion of murine primordial follicle reserve by gonadotropin-releasing hormone antagonists. Fertil Steril. 2005;83:1333-1338. - PubMed
-
- Peng P, Yang DZ, Zheng CY, Mo YQ, He YM, Zhang QX. Effects of gonadotropin releasing hormone analogues on chemotherapy-induced ovarian function damage in rats. Zhonghua Fu Chan Ke Za Zhi. 2007;42:546-550. - PubMed
-
- Gupta RK, Flaws JA. Gonadotropin-releasing hormone (GnRH) analogues and the ovary: do GnRH antagonists destroy primordial follicles? Fertil Steril. 2005;83:1339-1342. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

