Microbial biosynthesis of lactate esters
- PMID: 31548868
- PMCID: PMC6753613
- DOI: 10.1186/s13068-019-1563-z
Microbial biosynthesis of lactate esters
Abstract
Background: Green organic solvents such as lactate esters have broad industrial applications and favorable environmental profiles. Thus, manufacturing and use of these biodegradable solvents from renewable feedstocks help benefit the environment. However, to date, the direct microbial biosynthesis of lactate esters from fermentable sugars has not yet been demonstrated.
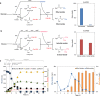
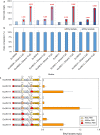
Results: In this study, we present a microbial conversion platform for direct biosynthesis of lactate esters from fermentable sugars. First, we designed a pyruvate-to-lactate ester module, consisting of a lactate dehydrogenase (ldhA) to convert pyruvate to lactate, a propionate CoA-transferase (pct) to convert lactate to lactyl-CoA, and an alcohol acyltransferase (AAT) to condense lactyl-CoA and alcohol(s) to make lactate ester(s). By generating a library of five pyruvate-to-lactate ester modules with divergent AATs, we screened for the best module(s) capable of producing a wide range of linear, branched, and aromatic lactate esters with an external alcohol supply. By co-introducing a pyruvate-to-lactate ester module and an alcohol (i.e., ethanol, isobutanol) module into a modular Escherichia coli (chassis) cell, we demonstrated for the first time the microbial biosynthesis of ethyl and isobutyl lactate esters directly from glucose. In an attempt to enhance ethyl lactate production as a proof-of-study, we re-modularized the pathway into (1) the upstream module to generate the ethanol and lactate precursors and (2) the downstream module to generate lactyl-CoA and condense it with ethanol to produce the target ethyl lactate. By manipulating the metabolic fluxes of the upstream and downstream modules through plasmid copy numbers, promoters, ribosome binding sites, and environmental perturbation, we were able to probe and alleviate the metabolic bottlenecks by improving ethyl lactate production by 4.96-fold. We found that AAT is the most rate-limiting step in biosynthesis of lactate esters likely due to its low activity and specificity toward the non-natural substrate lactyl-CoA and alcohols.
Conclusions: We have successfully established the biosynthesis pathway of lactate esters from fermentable sugars and demonstrated for the first time the direct fermentative production of lactate esters from glucose using an E. coli modular cell. This study defines a cornerstone for the microbial production of lactate esters as green solvents from renewable resources with novel industrial applications.
Keywords: Acetate ester; Alcohol acyltransferase; Escherichia coli; Ester; Ethyl lactate; Green solvent; Isobutyl lactate; Lactate ester; Modular cell.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures



References
-
- Kerton FM. Alternative solvents for green chemistry. Cambridge: RSC Pub; 2009.
-
- Lancaster M. Green chemistry: an introductory text. Cambridge: Royal Society of Chemistry; 2002.
-
- Aparicio S, Halajian S, Alcalde R, Garcia B, Leal JM. Liquid structure of ethyl lactate, pure and water mixed, as seen by dielectric spectroscopy, solvatochromic and thermophysical studies. Chem Phys Lett. 2008;454(1–3):49–55. doi: 10.1016/j.cplett.2008.01.068. - DOI
-
- Paul S, Pradhan K, Das AR. Ethyl lactate as a green solvent: a promising bio-compatible media for organic synthesis. Curr Green Chem. 2016;3(1):111–118. doi: 10.2174/2213346103666151203203139. - DOI
-
- Weissermel K, Arpe H-J. Industrial organic chemistry. 3. Weinheim: VCH Publishers Inc.; 1997.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

