Inside-Out 3D Reversible Ion-Triggered Shape-Morphing Hydrogels
- PMID: 31549074
- PMCID: PMC6750057
- DOI: 10.34133/2019/6398296
Inside-Out 3D Reversible Ion-Triggered Shape-Morphing Hydrogels
Abstract
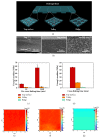
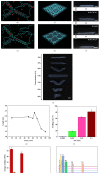
Shape morphing is a critical aptitude for the survival of organisms and is determined by anisotropic tissue composition and directional orientation of micro- and nanostructures within cell walls, resulting in different swelling behaviors. Recent efforts have been dedicated to mimicking the behaviors that nature has perfected over billions of years. We present a robust strategy for preparing 3D periodically patterned single-component sodium alginate hydrogel sheets cross-linked with Ca2+ ions, which can reversibly deform and be retained into various desirable inside-out shapes as triggered by biocompatible ions (Na+/Ca2+). By changing the orientations of the patterned microchannels or triggering with Na+/Ca2+ ions, various 3D twisting, tubular, and plant-inspired architectures can be facilely programmed. Not only can the transformation recover their initial shapes reversibly, but also it can keep the designated shapes without continuous stimuli. These inside-out 3D reversible ion-triggered hydrogel transformations shall inspire more attractive applications in tissue engineering, biomedical devices, and soft robotics fields.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Ionov L. Biomimetic hydrogel-based actuating systems. Advanced Functional Materials. 2013;23(36):4555–4570. doi: 10.1002/adfm.201203692. - DOI
-
- Peng X., Liu T., Zhang Q., Shang C., Bai Q., Wang H. Surface patterning of hydrogels for programmable and complex shape deformations by ion inkjet printing. Advanced Functional Materials. 2017;27(33):p. 1701962. doi: 10.1002/adfm.201701962. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

