Systemic Tumor Necrosis Factor-Alpha Trajectories Relate to Brain Health in Typically Aging Older Adults
- PMID: 31549145
- PMCID: PMC7457183
- DOI: 10.1093/gerona/glz209
Systemic Tumor Necrosis Factor-Alpha Trajectories Relate to Brain Health in Typically Aging Older Adults
Abstract
Background: Central nervous system levels of tumor necrosis factor-alpha (TNF-α), a pro-inflammatory cytokine, regulate the neuroinflammatory response and may play a role in age-related neurodegenerative diseases. The longitudinal relation between peripheral levels of TNF-α and typical brain aging is understudied. We hypothesized that within-person increases in systemic TNF-α would track with poorer brain health outcomes in functionally normal adults.
Methods: Plasma-based TNF-α concentrations (pg/mL; fasting morning draws) and magnetic resonance imaging were acquired in 424 functionally intact adults (mean age = 71) followed annually for up to 8.4 years (mean follow-up = 2.2 years). Brain outcomes included total gray matter volume and white matter hyperintensities. Cognitive outcomes included composites of memory, executive functioning, and processing speed, as well as Mini-Mental State Examination total scores. Longitudinal mixed-effects models were used, controlling for age, sex, education, and total intracranial volume, as appropriate.
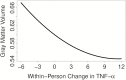
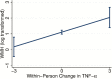
Results: TNF-α concentrations significantly increased over time (p < .001). Linear increases in within-person TNF-α were longitudinally associated with declines in gray matter volume (p < .001) and increases in white matter hyperintensities (p = .003). Exploratory analyses suggested that the relation between TNF-α and gray matter volume was curvilinear (TNF-α 2p = .002), such that initial increases in inflammation were associated with more precipitous atrophy. There was a negative linear relationship of within-person changes in TNF-α to Mini-Mental State Examination scores over time (p = .036) but not the cognitive composites (all ps >.05).
Conclusion: Systemic inflammation, as indexed by plasma TNF-α, holds potential as a biomarker for age-related declines in brain health.
Keywords: Brain aging; Cognition; Gray matter volume; Inflammation; Neuroimaging.
© The Author(s) 2019. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment.Brain Struct Funct. 2016 May;221(4):2135-46. doi: 10.1007/s00429-015-1034-7. Epub 2015 Apr 2. Brain Struct Funct. 2016. PMID: 25833685 Free PMC article.
-
Multiple Brain Markers are Linked to Age-Related Variation in Cognition.Cereb Cortex. 2016 Apr;26(4):1388-400. doi: 10.1093/cercor/bhu238. Epub 2014 Oct 14. Cereb Cortex. 2016. PMID: 25316342 Free PMC article.
-
Inflammatory biomarkers and brain health indicators in children with overweight and obesity: The ActiveBrains project.Brain Behav Immun. 2019 Oct;81:588-597. doi: 10.1016/j.bbi.2019.07.020. Epub 2019 Jul 19. Brain Behav Immun. 2019. PMID: 31330300
-
White matter hyperintensities mediate gray matter volume and processing speed relationship in cognitively unimpaired participants.Hum Brain Mapp. 2020 Apr 1;41(5):1309-1322. doi: 10.1002/hbm.24877. Epub 2019 Nov 28. Hum Brain Mapp. 2020. PMID: 31778002 Free PMC article.
-
Cognitive aging is not created equally: differentiating unique cognitive phenotypes in "normal" adults.Neurobiol Aging. 2019 May;77:13-19. doi: 10.1016/j.neurobiolaging.2019.01.007. Epub 2019 Jan 24. Neurobiol Aging. 2019. PMID: 30772736 Free PMC article.
Cited by
-
The role of dynapenia and obesity on cognitive function in older adults.Clin Nutr ESPEN. 2024 Oct;63:191-196. doi: 10.1016/j.clnesp.2024.06.039. Epub 2024 Jun 26. Clin Nutr ESPEN. 2024. PMID: 38963765 Free PMC article.
-
Astrocytes in aging.Neuron. 2025 Jan 8;113(1):109-126. doi: 10.1016/j.neuron.2024.12.010. Neuron. 2025. PMID: 39788083 Review.
-
Preliminary Evidence for a Relationship between Elevated Plasma TNFα and Smaller Subcortical White Matter Volume in HCV Infection Irrespective of HIV or AUD Comorbidity.Int J Mol Sci. 2021 May 7;22(9):4953. doi: 10.3390/ijms22094953. Int J Mol Sci. 2021. PMID: 34067023 Free PMC article.
-
Increased brain cytokine level associated impairment of vigilance and memory in aged rats can be alleviated by alpha7 nicotinic acetylcholine receptor agonist treatment.Geroscience. 2024 Feb;46(1):645-664. doi: 10.1007/s11357-023-01019-6. Epub 2023 Nov 23. Geroscience. 2024. PMID: 37994990 Free PMC article.
-
Necroptosis increases with age in the brain and contributes to age-related neuroinflammation.Geroscience. 2021 Oct;43(5):2345-2361. doi: 10.1007/s11357-021-00448-5. Epub 2021 Sep 13. Geroscience. 2021. PMID: 34515928 Free PMC article.
References
-
- Franceschi C, Bonafè M, Valensin S, et al. . Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x - PubMed
-
- McNerlan SE, Ross OA, Maeve Rea I.. Cytokine Expression and Production Changes in Very Old Age. Cham, Switzerland: Springer International Publishing; 2018. doi: 10.1007/978-3-319-64597-1_40-1
-
- Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2016;72:1218–1225. doi: 10.1093/gerona/glw240 - PubMed
-
- Fenn AM, Norden DM, Godbout JP.. Neuroinflammation in Aging. Cham, Switzerland: Springer International Publishing; 2015. doi: 10.1002/9781118732748.ch6
-
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011 - PMC - PubMed

