Determination of Cytochrome P450 Isoenzyme 2D6 (CYP2D6) Genotypes and Pharmacogenomic Impact on Primaquine Metabolism in an Active-Duty US Military Population
- PMID: 31549155
- PMCID: PMC6804407
- DOI: 10.1093/infdis/jiz386
Determination of Cytochrome P450 Isoenzyme 2D6 (CYP2D6) Genotypes and Pharmacogenomic Impact on Primaquine Metabolism in an Active-Duty US Military Population
Erratum in
-
Corrigendum to: Determination of Cytochrome P450 Isoenzyme 2D6 (CYP2D6) Genotypes and Pharmacogenomic Impact on Primaquine Metabolism in an Active-Duty US Military Population.J Infect Dis. 2020 Mar 16;221(7):1204. doi: 10.1093/infdis/jiz599. J Infect Dis. 2020. PMID: 31773155 Free PMC article. No abstract available.
Abstract
Background: Plasmodium vivax malaria requires a 2-week course of primaquine (PQ) for radical cure. Evidence suggests that the hepatic isoenzyme cytochrome P450 2D6 (CYP2D6) is the key enzyme required to convert PQ into its active metabolite.
Methods: CYP2D6 genotypes and phenotypes of 550 service personnel were determined, and the pharmacokinetics (PK) of a 30-mg oral dose of PQ was measured in 45 volunteers. Blood and urine samples were collected, with PQ and metabolites were measured using ultraperformance liquid chromatography with mass spectrometry.
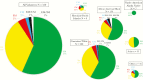
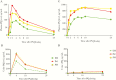
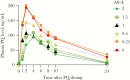
Results: Seventy-six CYP2D6 genotypes were characterized for 530 service personnel. Of the 515 personnel for whom a single phenotype was predicted, 58% had a normal metabolizer (NM) phenotype, 35% had an intermediate metabolizer (IM) phenotype, 5% had a poor metabolizer (PM) phenotype, and 2% had an ultrametabolizer phenotype. The median PQ area under the concentration time curve from 0 to ∞ was lower for the NM phenotype as compared to the IM or PM phenotypes. The novel 5,6-ortho-quinone was detected in urine but not plasma from all personnel with the NM phenotype.
Conclusion: The plasma PK profile suggests PQ metabolism is decreased in personnel with the IM or PM phenotypes as compared to those with the NM phenotype. The finding of 5,6-ortho-quinone, the stable surrogate for the unstable 5-hydroxyprimaquine metabolite, almost exclusively in personnel with the NM phenotype, compared with sporadic or no production in those with the IM or PM phenotypes, provides further evidence for the role of CYP2D6 in radical cure.
Clinical trials registration: NCT02960568.
Keywords: 5,6-ortho-quinone; CYP2D6; Primaquine; genotype; metabolism; military; pharmacokinetics; phenotype.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- World Health Organization (WHO), World malaria report 2017. Geneva: World Health Organization, 2017.
-
- Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 2006; 75:402–15. - PubMed
-
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis 2004; 39:1336–45. - PubMed
-
- Bennett JW, Pybus BS, Yadava A, et al. . Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 2013; 369:1381–2. - PubMed
-
- Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008; 83:234–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

