Therapeutic Drug Monitoring of Biologics During Induction to Prevent Primary Non-Response
- PMID: 31549158
- PMCID: PMC7392326
- DOI: 10.1093/ecco-jcc/jjz162
Therapeutic Drug Monitoring of Biologics During Induction to Prevent Primary Non-Response
Abstract
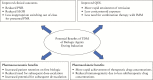
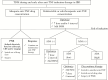
Biologic therapies have revolutionized the management of inflammatory bowel disease [IBD], but primary and secondary non-responses occur in a significant proportion of patients. Therapeutic drug monitoring [TDM] now has an established role in the treatment algorithm for managing secondary loss of response to anti-tumour necrosis factor [anti-TNF] agents during maintenance therapy. Data to support the use of TDM in the management of secondary loss of response to vedolizumab and ustekinumab are emerging. The potential to prevent primary non-response to biologic agents during induction is of equal, and potentially greater, clinical importance. Again, most data supporting the use of 'proactive' TDM during induction pertains to the use of anti-TNF agents, but signals of efficacy for the use of TDM during induction with other biologic classes are now appearing. This review aims to summarize data on the use of TDM during induction to prevent pharmacokinetic primary non-response to all three classes of biologic therapy currently available for the treatment of IBD.
Keywords: induction; primary non-response; therapeutic drug monitoring.
Copyright © 2019 European Crohn’s and Colitis Organisation (ECCO). Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43:317–33. - PubMed
-
- Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1245–55 e8. - PubMed
-
- Schnitzler F, Fidder H, Ferrante M, et al. .. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. - PubMed
-
- Colombel JF, Panaccione R, Bossuyt P, et al. .. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. - PubMed