The BRCA2 mutation status shapes the immune phenotype of prostate cancer
- PMID: 31549213
- PMCID: PMC6805809
- DOI: 10.1007/s00262-019-02393-x
The BRCA2 mutation status shapes the immune phenotype of prostate cancer
Abstract
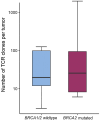
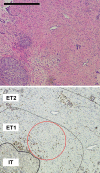
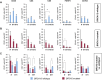
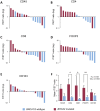
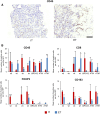
Defects in DNA damage repair caused by mutations in BRCA1/2, ATM or other genes have been shown to play an important role in the development and progression of prostate cancer. The influence of such mutations on anti-tumor immunity in prostate cancer, however, is largely unknown. To better understand the correlation between BRCA1/2 mutations and the immune phenotype in prostate cancer, we characterized the immune infiltrate of eight BRCA2-mutated tumors in comparison with eight BRCA1/2 wild-type patients by T-cell receptor sequencing and immunohistochemistry for CD45, CD4, CD8, FOXP3, and CD163. In addition, we analyzed seven prostate cancer biopsies that were either BRCA2 or ATM-mutated in comparison with wild-type tumors. Whereas in BRCA1/2 wild-type tumors, immune cells were found predominantly extratumorally, most BRCA2-mutated tumors including one biopsy showed a significantly increased intratumoral immune cell infiltration. The ratio of intratumoral to extratumoral immune cells was considerably higher in BRCA2-mutated tumors for all markers and reached statistical significance for CD4 (p = 0.007), CD8 (p = 0.006), and FOXP3 (p = 0.001). However, the intratumoral CD8 to FOXP3 ratio showed a trend to be lower in BRCA2-mutated tumors suggesting a more suppressed tumor immune microenvironment. Our findings provide a rationale for the future use of immune oncological approaches in BRCA2-mutated prostate cancer and may encourage efforts to target immunosuppressive T-cell populations to prime tumors for immunotherapy.
Keywords: BRCA1/2; Homologous recombination deficiency; Immune checkpoint inhibitors; Prostate cancer; Tumor microenvironment; Tumor-infiltrating lymphocytes.
Conflict of interest statement
The authors declare that they have no potential conflict of interest.
Figures


References
-
- Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, Elemento O, Rubin MA, Robinson D, Lonigro R, Hussain M, Chinnaiyan A, Vinson J, Filipenko J, Garraway L, Taplin ME, AlDubayan S, Han GC, Beightol M, Morrissey C, Nghiem B, Cheng HH, Montgomery B, Walsh T, Casadei S, Berger M, Zhang L, Zehir A, Vijai J, Scher HI, Sawyers C, Schultz N, Kantoff PW, Solit D, Robson M, Van Allen EM, Offit K, de Bono J, Nelson PS. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453. doi: 10.1056/NEJMoa1603144. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

