Evaluation of the Feasibility of a Web-Based Survey to Assess Patient-Reported Symptom Improvement and Treatment Satisfaction Among Patients with Psoriatic Arthritis Receiving Secukinumab
- PMID: 31549346
- PMCID: PMC6842331
- DOI: 10.1007/s40261-019-00856-8
Evaluation of the Feasibility of a Web-Based Survey to Assess Patient-Reported Symptom Improvement and Treatment Satisfaction Among Patients with Psoriatic Arthritis Receiving Secukinumab
Abstract
Background and objective: Patient perspectives regarding treatment experience and satisfaction may be useful for clinicians when making treatment strategies. This US-based study assessed the feasibility of evaluating real-world, patient-reported narratives regarding symptom improvement and treatment satisfaction among patients with psoriatic arthritis treated with secukinumab.
Methods: A cross-sectional, web-based survey collected data on demographics, disease characteristics, symptoms before and after secukinumab use, and treatment satisfaction with secukinumab.
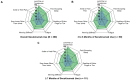
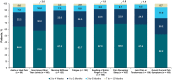
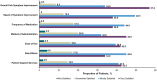
Results: Of 2755 patients screened, 200 patients with psoriatic arthritis were eligible and included in the analysis. Their mean age was 36.0 (standard deviation, 10.0) years; 55.5% were male and 75.0% were white. Most (87.5%) were biologic experienced; the primary reason for discontinuation of their previous treatment was lack of effectiveness (28.6%). Most patients (79.9%) reported overall psoriatic arthritis symptom improvement after secukinumab initiation compared with before secukinumab initiation; a similar trend was observed for all individual symptoms evaluated. Approximately half of patients reported improvement within 4 weeks after starting secukinumab treatment, and > 90% reported improvement within 6 months. Most patients (≥ 96%) expressed overall satisfaction with secukinumab regarding symptom improvement, speed of symptom improvement, frequency of administration, method of administration, ease of use, patient support services, and side effects, if any.
Conclusions: Patient-reported perspectives may be feasibly collected to provide insights into treatment experience and satisfaction of secukinumab. Most patients with psoriatic arthritis in our real-world study experienced symptom improvement after initiating secukinumab; > 50% of patients reported symptom improvement within 4 weeks. Additionally, almost all patients reported satisfaction with secukinumab treatment.
Conflict of interest statement
Marina Magrey has served as a consultant for Novartis. Michael Bozyczko has no conflicts of interest that are directly relevant to the content of this article. Daniel Wolin, Margaret Mordin, Lori McLeod, Eric Davenport, and Costel Chirila are employees of RTI Health Solutions. Peter Hur is an employee of Novartis.
Figures
Similar articles
-
A Pilot Study to Assess the Feasibility of a Web-Based Survey to Examine Patient-Reported Symptoms and Satisfaction in Patients with Ankylosing Spondylitis Receiving Secukinumab.Drugs Real World Outcomes. 2019 Jun;6(2):83-91. doi: 10.1007/s40801-019-0154-4. Drugs Real World Outcomes. 2019. PMID: 31054047 Free PMC article.
-
Real world effectiveness and satisfaction with secukinumab in the treatment of patients with psoriatic arthritis: a population survey in five European countries.Curr Med Res Opin. 2021 Oct;37(10):1845-1853. doi: 10.1080/03007995.2021.1954500. Epub 2021 Jul 23. Curr Med Res Opin. 2021. PMID: 34256669
-
Treatment of psoriatic arthritis with secukinumab: a case series.J Dermatolog Treat. 2018;29(sup1):6-8. doi: 10.1080/09546634.2018.1527994. J Dermatolog Treat. 2018. PMID: 30247936
-
Matching-adjusted indirect comparison: secukinumab versus infliximab in biologic-naive patients with psoriatic arthritis.J Comp Eff Res. 2019 May;8(7):497-510. doi: 10.2217/cer-2018-0141. Epub 2019 Feb 26. J Comp Eff Res. 2019. PMID: 30806520
-
Association of previous treatment with anti-tumour necrosis factor inhibitors with the effectiveness of secukinumab in the treatment of psoriatic arthritis: systematic review and meta-analysis.Rheumatology (Oxford). 2020 Dec 1;59(12):3657-3665. doi: 10.1093/rheumatology/keaa449. Rheumatology (Oxford). 2020. PMID: 33038239
Cited by
-
Evaluating patient-reported adherence and outcomes in specialty disease states: A dual-site initiative.J Manag Care Spec Pharm. 2024 Jul;30(7):710-718. doi: 10.18553/jmcp.2024.30.7.710. J Manag Care Spec Pharm. 2024. PMID: 38950163 Free PMC article.
-
Secukinumab: A Review in Psoriatic Arthritis.Drugs. 2021 Mar;81(4):483-494. doi: 10.1007/s40265-021-01476-3. Epub 2021 Mar 4. Drugs. 2021. PMID: 33661486 Free PMC article. Review.
-
Real-world effectiveness and persistence of secukinumab in the treatment of patients with psoriatic arthritis.Front Med (Lausanne). 2023 Nov 20;10:1294247. doi: 10.3389/fmed.2023.1294247. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38053615 Free PMC article.
-
Effectiveness and safety of secukinumab in 608 patients with psoriatic arthritis in real life: a 24-month prospective, multicentre study.RMD Open. 2021 Feb;7(1):e001519. doi: 10.1136/rmdopen-2020-001519. RMD Open. 2021. PMID: 33593933 Free PMC article.
-
Secukinumab after first-line tumor necrosis factor-alpha inhibitor therapy in psoriatic arthritis: A real-world retrospective cohort study.Arch Rheumatol. 2024 Jan 31;39(1):71-80. doi: 10.46497/ArchRheumatol.2024.10050. eCollection 2024 Mar. Arch Rheumatol. 2024. PMID: 38774692 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

