Elucidation of the Structure of a Thiol Functionalized Cu-tmpa Complex Anchored to Gold via a Self-Assembled Monolayer
- PMID: 31549820
- PMCID: PMC6784813
- DOI: 10.1021/acs.inorgchem.9b01921
Elucidation of the Structure of a Thiol Functionalized Cu-tmpa Complex Anchored to Gold via a Self-Assembled Monolayer
Abstract
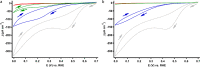
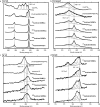
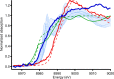
The structure of the copper complex of the 6-((1-butanethiol)oxy)-tris(2-pyridylmethyl)amine ligand (Cu-tmpa-O(CH2)4SH) anchored to a gold surface has been investigated. To enable covalent attachment of the complex to the gold surface, a heteromolecular self-assembled monolayer (SAM) of butanethiol and a thiol-substituted tmpa ligand was used. Subsequent formation of the immobilized copper complex by cyclic voltammetry in the presence of Cu(OTf)2 resulted in the formation of the anchored Cu-tmpa-O(CH2)4SH system which, according to scanning electron microscopy and X-ray diffraction, did not contain any accumulated copper nanoparticles or crystalline copper material. Electrochemical investigation of the heterogenized system barely showed any redox activity and lacked the typical CuII/I redox couple in contrast to the homogeneous complex in solution. The difference between the heterogenized system and the homogeneous complex was confirmed by X-ray photoelectron spectroscopy; the XPS spectrum did not show any satellite features of a CuII species but instead showed the presence of a CuI ion in a ∼2:3 ratio to nitrogen and a ∼2:7 ratio to sulfur. The +I oxidation state of the copper species was confirmed by the edge position in the X-ray absorption near-edge structure (XANES) region of the X-ray absorption spectrum. These results show that upon immobilization of Cu-tmpa-O(CH2)4SH, the resulting structure is not identical to the homogeneous CuII-tmpa complex. Upon anchoring, a novel CuI species is formed instead. This illustrates the importance of a thorough characterization of heterogenized molecular systems before drawing any conclusions regarding the structure-function relationships.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
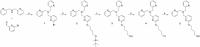




Similar articles
-
Copper(I) complex O(2)-reactivity with a N(3)S thioether ligand: a copper-dioxygen adduct including sulfur ligation, ligand oxygenation, and comparisons with all nitrogen ligand analogues.Inorg Chem. 2007 Jul 23;46(15):6056-68. doi: 10.1021/ic700541k. Epub 2007 Jun 20. Inorg Chem. 2007. PMID: 17580938
-
Copper(I)/S(8) reversible reactions leading to an end-on bound dicopper(II) disulfide complex: nucleophilic reactivity and analogies to copper-dioxygen chemistry.J Am Chem Soc. 2007 Jul 18;129(28):8882-92. doi: 10.1021/ja071968z. Epub 2007 Jun 26. J Am Chem Soc. 2007. PMID: 17592845
-
Synthesis, Structure, and Solution NMR Studies of Cyanide-Copper(II) and Cyanide-Bridged Iron(III)-Copper(II) Complexes.Inorg Chem. 1999 Mar 8;38(5):848-858. doi: 10.1021/ic981091s. Inorg Chem. 1999. PMID: 11670854
-
Influence of Ligand Denticity and Flexibility on the Molecular Copper Mediated Oxygen Reduction Reaction.Inorg Chem. 2020 Nov 16;59(22):16398-16409. doi: 10.1021/acs.inorgchem.0c02204. Epub 2020 Oct 27. Inorg Chem. 2020. PMID: 33108871 Free PMC article.
-
Generation and characterization of [(P)M-(X)-Co(TMPA)]n+ assemblies; P = Porphyrinate, M = FeIII and CoIII, X = O2-, OH-, O2(2-), and TMPA = tris(2-pyridylmethyl)amine.Inorg Chem. 2007 Apr 16;46(8):3017-26. doi: 10.1021/ic061686k. Epub 2007 Mar 20. Inorg Chem. 2007. PMID: 17371009
Cited by
-
A Heterogenized Copper Phenanthroline System to Catalyze the Oxygen Reduction Reaction.ChemElectroChem. 2022 Feb 11;9(3):e202101365. doi: 10.1002/celc.202101365. Epub 2022 Feb 2. ChemElectroChem. 2022. PMID: 35911790 Free PMC article.
-
The Role of Metal-Organic Framework Induced Confinement Effects on Molecular Electrocatalysts Relevant to the Energy Transition.ChemSusChem. 2025 Jun 17;18(12):e202402676. doi: 10.1002/cssc.202402676. Epub 2025 Apr 24. ChemSusChem. 2025. PMID: 40272071 Free PMC article. Review.
References
-
- Amao Y. Probes and polymers for optical sensing of oxygen. Microchim. Acta 2003, 143, 1–12. 10.1007/s00604-003-0037-x. - DOI
-
- Machini W. B. S.; Teixeira M. F. S. Electrochemical properties of the oxo-manganese-phenanthroline complex immobilized on ion-exchange polymeric film and its application as biomimetic sensor for sulfite ions. Electroanalysis 2014, 26, 2182–2190. 10.1002/elan.201400289. - DOI
-
- Mayuri P.; Saravanan N.; Senthil Kumar A. A bioinspired copper 2,2-bipyridyl complex immobilized mwcnt modified electrode prepared by a new strategy for elegant electrocatalytic reduction and sensing of hydrogen peroxide. Electrochim. Acta 2017, 240, 522–533. 10.1016/j.electacta.2017.04.082. - DOI
LinkOut - more resources
Full Text Sources

