A prospective, iterative, adaptive trial of carfilzomib-based desensitization
- PMID: 31550069
- PMCID: PMC7872208
- DOI: 10.1111/ajt.15613
A prospective, iterative, adaptive trial of carfilzomib-based desensitization
Abstract
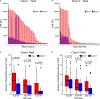
Proteasome inhibitor-based strategies hold promise in transplant but have yielded varying results. Carfilzomib, a second-generation proteasome inhibitor, may possess advantages over bortezomib, the first-generation proteasome inhibitors. The purpose of this study was to evaluate the safety, toxicity, and preliminary efficacy of carfilzomib in highly HLA-sensitized kidney transplant candidates. Renal transplant candidates received escalating doses of carfilzomib followed by plasmapheresis (group A) or an identical regimen with additional plasmapheresis once weekly before carfilzomib dosing. Thirteen participants received carfilzomib, which was well tolerated with most adverse events classified as low grade. The safety profile was similar to bortezomib desensitization; however, neurotoxicity was not observed with carfilzomib. Toxicity resulted in permanent dose reduction in 1 participant but caused no withdrawals or deaths. HLA antibodies were substantially reduced with carfilzomib alone, and median maximal immunodominant antibody reduction was 72.8% (69.8% for group A, P = .031, 80.1% for group B, P = .938). After depletion, rebound occurred rapidly and antibody levels returned to baseline between days 81 and 141. Bone marrow studies revealed that approximately 69.2% of plasma cells were depleted after carfilzomib monotherapy. Carfilzomib monotherapy-based desensitization provides an acceptable safety and toxicity profile while leading to significant bone marrow plasma cell depletion and anti-HLA antibody reduction.
Keywords: alloantibody; clinical research/practice; clinical trial; desensitization; histocompatibility; immunosuppression/immune modulation; kidney transplantation/nephrology; panel reactive antibody (PRA); plasma cells; translational research/science.
© 2019 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures



References
-
- Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348–357. - PubMed
-
- Bostock IC, Alberú J, Arvizu A, et al. Probability of deceased donor kidney transplantation based on % PRA. Transpl Immunol. 2013;28:154–158. - PubMed
-
- Alachkar N, Lonze BE, Zachary AA, et al. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte antigen antibodies in highly sensitized patients. Transplantation. 2012;94:165–171. - PubMed
-
- Marfo K, Ling M, Bao YI, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation. 2012;94:345–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

