Optimizing bacterial DNA extraction in urine
- PMID: 31550285
- PMCID: PMC6759279
- DOI: 10.1371/journal.pone.0222962
Optimizing bacterial DNA extraction in urine
Abstract
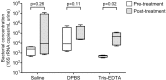
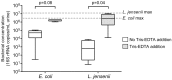
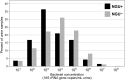
Urine is an acceptable, non-invasive sample for investigating the human urogenital microbiota and for the diagnosis of sexually transmitted infections. However, low quantities of bacterial DNA and PCR inhibitors in urine may prevent efficient PCR amplification for molecular detection of bacteria. Furthermore, cold temperatures used to preserve DNA and bacteria in urine can promote precipitation of crystals that interfere with DNA extraction. Saline, Dulbecco's Phosphate Buffered Saline, or Tris-EDTA buffer were added to urine from adult men to determine if crystal precipitation could be reversed without heating samples beyond ambient temperature. Total bacterial DNA concentrations and PCR inhibition were measured using quantitative PCR assays to compare DNA yields with and without buffer addition. Dissolution of crystals with Tris-EDTA prior to urine centrifugation was most effective in increasing bacterial DNA recovery and reducing PCR inhibition. DNA recovery using Tris-EDTA was further tested by spiking urine with DNA from bacterial isolates and median concentrations of Lactobacillus jensenii and Escherichia coli 16S rRNA gene copies were found to be higher in urine processed with Tris-EDTA. Maximizing bacterial DNA yield from urine may facilitate more accurate assessment of bacterial populations and increase detection of specific bacteria in the genital tract.
Conflict of interest statement
LEM has received donations of test kits, equipment, reagents, and speaking honoraria from Hologic Incorporated. The company had no role in the research presented in this paper and this does not alter our adherence to PLOS ONE policies on sharing data and materials. All other authors report no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

