Entrainment of the Circadian Clock of the Enteric Bacterium Klebsiella aerogenes by Temperature Cycles
- PMID: 31551197
- PMCID: PMC6831877
- DOI: 10.1016/j.isci.2019.09.007
Entrainment of the Circadian Clock of the Enteric Bacterium Klebsiella aerogenes by Temperature Cycles
Abstract
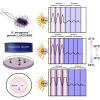
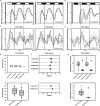
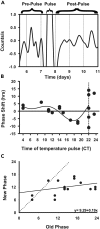
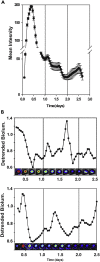
The gastrointestinal bacterium Klebsiella (née Enterobacter) aerogenes expresses an endogenously generated, temperature-compensated circadian rhythm in swarming motility. We hypothesized that this rhythm may be synchronized/entrained in vivo by body temperature (TB). To determine entrainment, cultures expressing bioluminescence were exposed to temperature cycles of 1°C (35°C-36°C) or 3°C (34°C-37°C) in amplitude at periods (T-cycles) of T = 22, T = 24, or T = 28 h. Bacteria entrained to all T-cycles at both amplitudes and with stable phase relationships. A high-amplitude phase response curve (PRC) in response to 1-h pulses of 3°C temperature spike (34°C-37°C) at different circadian phases was constructed, revealing a Type-0 phase resetting paradigm. Furthermore, real-time bioluminescence imaging revealed a spatiotemporal pattern to the circadian rhythm. These data are consistent with the hypothesis that the K. aerogenes circadian clock entrains to its host via detection of and phase shifting to the daily pattern of TB.
Keywords: Biological Sciences; Chronobiology; Microbiology.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Aschoff J. Human circadian rhythms in activity, body temperature and other functions. Life Sci. Space Res. 1967;5:159–173. - PubMed
-
- Baehr E.K., Revelle W., Eastman C.I. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J. Sleep Res. 2000;9:117–127. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

