Harnessing Ion-Binding Sites for GPCR Pharmacology
- PMID: 31551350
- PMCID: PMC6782022
- DOI: 10.1124/pr.119.017863
Harnessing Ion-Binding Sites for GPCR Pharmacology
Abstract
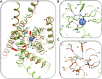
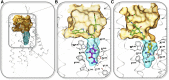
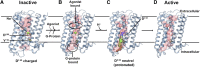
Endogenous ions play important roles in the function and pharmacology of G-protein coupled receptors (GPCRs). Historically the evidence for ionic modulation of GPCR function dates to 1973 with studies of opioid receptors, where it was demonstrated that physiologic concentrations of sodium allosterically attenuated agonist binding. This Na+-selective effect was distinct from effects of other monovalent and divalent cations, with the latter usually counteracting sodium's negative allosteric modulation of binding. Since then, numerous studies documenting the effects of mono- and divalent ions on GPCR function have been published. While ions can act selectively and nonselectively at many sites in different receptors, the discovery of the conserved sodium ion site in class A GPCR structures in 2012 revealed the unique nature of Na+ site, which has emerged as a near-universal site for allosteric modulation of class A GPCR structure and function. In this review, we synthesize and highlight recent advances in the functional, biophysical, and structural characterization of ions bound to GPCRs. Taken together, these findings provide a molecular understanding of the unique roles of Na+ and other ions as GPCR allosteric modulators. We will also discuss how this knowledge can be applied to the redesign of receptors and ligand probes for desired functional and pharmacological profiles. SIGNIFICANCE STATEMENT: The function and pharmacology of GPCRs strongly depend on the presence of mono and divalent ions in experimental assays and in living organisms. Recent insights into the molecular mechanism of this ion-dependent allosterism from structural, biophysical, biochemical, and computational studies provide quantitative understandings of the pharmacological effects of drugs in vitro and in vivo and open new avenues for the rational design of chemical probes and drug candidates with improved properties.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


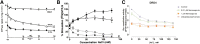
References
-
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. (2006) Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature 444:106–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

