NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling
- PMID: 31551515
- PMCID: PMC8115140
- DOI: 10.1038/s41423-019-0296-z
NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling
Abstract
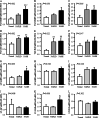
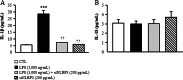
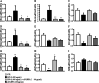
The NLRP3-IL-1β pathway plays an important role in adipose tissue (AT)-induced inflammation and the development of obesity-associated comorbidities. We aimed to determine the impact of NLRP3 on obesity and its associated metabolic alterations as well as its role in adipocyte inflammation and extracellular matrix (ECM) remodeling. Samples obtained from 98 subjects were used in a case-control study. The expression of different components of the inflammasome as well as their main effectors and inflammation- and ECM remodeling-related genes were analyzed. The impact of blocking NLRP3 using siRNA in lipopolysaccharide (LPS)-mediated inflammation and ECM remodeling signaling pathways was evaluated. We demonstrated that obesity (P < 0.01), obesity-associated T2D (P < 0.01) and NAFLD (P < 0.05) increased the expression of different components of the inflammasome as well as the expression and release of IL-1β and IL-18 in AT. We also found that obese patients with T2D exhibited increased (P < 0.05) hepatic gene expression levels of NLRP3, IL1B and IL18. We showed that NLRP3, but not NLRP1, is regulated by inflammation and hypoxia in visceral adipocytes. We revealed that the inhibition of NLRP3 in human visceral adipocytes significantly blocked (P < 0.01) LPS-induced inflammation by downregulating the mRNA levels of CCL2, IL1B, IL6, IL8, S100A8, S100A9, TLR4 and TNF as well as inhibiting (P < 0.01) the secretion of IL1-β into the culture medium. Furthermore, blocking NLRP3 attenuated (P < 0.01) the LPS-induced expression of important molecules involved in AT fibrosis (COL1A1, COL4A3, COL6A3 and MMP2). These novel findings provide evidence that blocking the expression of NLRP3 reduces AT inflammation with significant fibrosis attenuation.
Keywords: Inflammasone; Inflammation; NLRP3; Nonalcoholic fatty liver disease; Obesity; Type 2 diabetes.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

