Translating neural stem cells to neurons in the mammalian brain
- PMID: 31551564
- PMCID: PMC7224185
- DOI: 10.1038/s41418-019-0411-9
Translating neural stem cells to neurons in the mammalian brain
Abstract
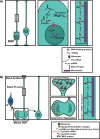
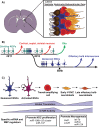
The mammalian neocortex underlies our perception of sensory information, performance of motor activities, and higher-order cognition. During mammalian embryogenesis, radial glial precursor cells sequentially give rise to diverse populations of excitatory cortical neurons, followed by astrocytes and oligodendrocytes. A subpopulation of these embryonic neural precursors persists into adulthood as neural stem cells, which give rise to inhibitory interneurons and glia. Although the intrinsic mechanisms instructing the genesis of these distinct progeny have been well-studied, most work to date has focused on transcriptional, epigenetic, and cell-cycle control. Recent studies, however, have shown that posttranscriptional mechanisms also regulate the cell fate choices of transcriptionally primed neural precursors during cortical development. These mechanisms are mediated primarily by RNA-binding proteins and microRNAs that coordinately regulate mRNA translation, stability, splicing, and localization. Together, these findings point to an extensive network of posttranscriptional control and provide insight into both normal cortical development and disease. They also add another layer of complexity to brain development and raise important biological questions for future investigation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

