Single-trial neural dynamics are dominated by richly varied movements
- PMID: 31551604
- PMCID: PMC6768091
- DOI: 10.1038/s41593-019-0502-4
Single-trial neural dynamics are dominated by richly varied movements
Abstract
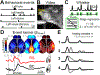
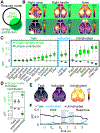
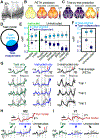
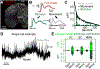
When experts are immersed in a task, do their brains prioritize task-related activity? Most efforts to understand neural activity during well-learned tasks focus on cognitive computations and task-related movements. We wondered whether task-performing animals explore a broader movement landscape and how this impacts neural activity. We characterized movements using video and other sensors and measured neural activity using widefield and two-photon imaging. Cortex-wide activity was dominated by movements, especially uninstructed movements not required for the task. Some uninstructed movements were aligned to trial events. Accounting for them revealed that neurons with similar trial-averaged activity often reflected utterly different combinations of cognitive and movement variables. Other movements occurred idiosyncratically, accounting for trial-by-trial fluctuations that are often considered 'noise'. This held true throughout task-learning and for extracellular Neuropixels recordings that included subcortical areas. Our observations argue that animals execute expert decisions while performing richly varied, uninstructed movements that profoundly shape neural activity.
Figures




References
Additional References
-
- Ratzlaff EH & Grinvald A A tandem-lens epifluorescence macroscope: hundred-fold brightness advantage for wide-field imaging. J. Neurosci. Methods 36, 127–137 (1991). - PubMed
-
- Pachitariu M et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. bioRxiv 061507 (2016). doi: 10.1101/061507 - DOI
-
- Jia H, Rochefort NL, Chen X & Konnerth A In vivo two-photon imaging of sensory-evoked dendritic calcium signals in cortical neurons. Nat Protoc 6, 28–35 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

