Generational Biodegradable and Regenerative Polyphosphazene Polymers and their Blends with Poly (lactic-co-glycolic acid)
- PMID: 31551636
- PMCID: PMC6758934
- DOI: 10.1016/j.progpolymsci.2019.101146
Generational Biodegradable and Regenerative Polyphosphazene Polymers and their Blends with Poly (lactic-co-glycolic acid)
Abstract
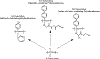
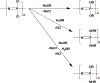
New fields such as regenerative engineering have driven the design of advanced biomaterials with a wide range of properties. Regenerative engineering is a multidisciplinary approach that integrates the fields of advanced materials science and engineering, stem cell science, physics, developmental biology, and clinical translation for the regeneration of complex tissues. The complexity and demands of this innovative approach have motivated the synthesis of new polymeric materials that can be customized to meet application-specific needs. Polyphosphazene polymers represent this fundamental change and are gaining renewed interest as biomaterials due to their outstanding synthetic flexibility, neutral bioactivity (buffering degradation products), and tunable properties across the range. Polyphosphazenes are a unique class of polymers composed of an inorganic backbone with alternating phosphorus and nitrogen atoms. Each phosphorus atom bears two substituents, with a wide variety of side groups available for property optimization. Polyphosphazenes have been investigated as potential biomaterials for regenerative engineering. Polyphosphazenes for use in regenerative applications have evolved as a class to include different generations of degradable polymers. The first generation of polyphosphazenes for tissue regeneration entailed the use of hydrolytically active side groups such as imidazole, lactate, glycolate, glucosyl, or glyceryl groups. These side groups were selected based on their ability to sensitize the polymer backbone to hydrolysis, which allowed them to break down into non-toxic small molecules that could be metabolized or excreted. The second generation of degradable polyphosphazenes developed consisted of polymers with amino acid ester side groups. When blended with poly (lactic acid-co-glycolic acid) (PLGA), the feasibility of neutralizing acidic degradation products of PLGA was demonstrated. The blends formed were mostly partially miscible. The desire to improve miscibility led to the design of the third generation of degradable polyphosphazenes by incorporating dipeptide side groups which impart significant hydrogen bonding capability to the polymer for the formation of completely miscible polyphosphazene-PLGA blends. Blend system of the dipeptide-based polyphosphazene and PLGA exhibit a unique degradation behavior that allows the formation of interconnected porous structures upon degradation. These inherent pore-forming properties have distinguished degradable polyphosphazenes as a potentially important class of biomaterials for further study. The design considerations and strategies for the different generations of degradable polyphosphazenes and future directions are discussed.
Keywords: biocompatibility; biodegradable polyphosphazene; biomaterials; poly (lactic-co-glycolic acid); regenerative engineering.
Conflict of interest statement
Competing interest The authors declare no competing financial interest.
Figures


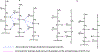



References
-
- Pillai C Recent advances in biodegradable polymeric materials. Mater Sci Technol 2014;30:558–66.
-
- Ogueri KS, Allcock HR, Laurencin CT. Polyphosphazene Polymer. Encycl Polym Sci Technol New York: John Wiley & Sons Inc, 2019;DOI: 10.1002/0471440264.pst284.pub2 (23 pp). - DOI
-
- Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med 2012;4:160–0. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
