Novel Optogenetic Approaches in Epilepsy Research
- PMID: 31551699
- PMCID: PMC6743373
- DOI: 10.3389/fnins.2019.00947
Novel Optogenetic Approaches in Epilepsy Research
Abstract
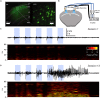
Epilepsy is a major neurological disorder characterized by repeated seizures afflicting 1% of the global population. The emergence of seizures is associated with several comorbidities and severely decreases the quality of life of patients. Unfortunately, around 30% of patients do not respond to first-line treatment using anti-seizure drugs (ASDs). Furthermore, it is still unclear how seizures arise in the healthy brain. Therefore, it is critical to have well developed models where a causal understanding of epilepsy can be investigated. While the development of seizures has been studied in several animal models, using chemical or electrical induction, deciphering the results of such studies has been difficult due to the uncertainty of the cell population being targeted as well as potential confounds such as brain damage from the procedure itself. Here we describe novel approaches using combinations of optical and genetic methods for studying epileptogenesis. These approaches can circumvent some shortcomings associated with the classical animal models and may thus increase the likelihood of developing new treatment options.
Keywords: animal models; epilepsy; kindling; optogenetics; plasticity; seizures.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

