New Insights Into Cholinergic Neuron Diversity
- PMID: 31551706
- PMCID: PMC6736589
- DOI: 10.3389/fnmol.2019.00204
New Insights Into Cholinergic Neuron Diversity
Abstract
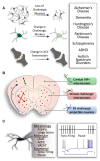
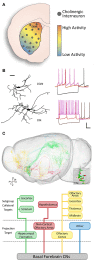
Cholinergic neurons comprise a small population of cells in the striatum but have fundamental roles in fine tuning brain function, and in the etiology of neurological and psychiatric disorders such as Parkinson's disease (PD) or schizophrenia. The process of developmental cell specification underlying neuronal identity and function is an area of great current interest. There has been significant progress in identifying the developmental origins, commonalities in molecular markers, and physiological properties of the cholinergic neurons. Currently, we are aware of a number of key factors that promote cholinergic fate during development. However, the extent of cholinergic cell diversity is still largely underestimated. New insights into the biological basis of their specification indicate that cholinergic neurons may be far more diverse than previously thought. This review article, highlights the physiological features and the synaptic properties that segregate cholinergic cell subtypes. It provides an accurate picture of cholinergic cell diversity underlying their organization and function in neuronal networks. This review article, also discusses current challenges in deciphering the logic of the cholinergic cell heterogeneity that plays a fundamental role in the control of neural processes in health and disease.
Keywords: acetylcholine; development; diversity; interneurons; striatum.
Figures

References
-
- Albert-Gascó H., García-Avilés A., Moustafa S., Sánchez-Sarasua S., Gundlach A. L., Olucha-Bordonau F. E., et al. (2017). Central relaxin-3 receptor (RXFP3) activation increases ERK phosphorylation in septal cholinergic neurons and impairs spatial working memory. Brain Struct. Funct. 222, 449–463. 10.1007/s00429-016-1227-8 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

