Hydroxycamptothecin Inhibits Peritendinous Adhesion via the Endoplasmic Reticulum Stress-Dependent Apoptosis
- PMID: 31551777
- PMCID: PMC6737834
- DOI: 10.3389/fphar.2019.00967
Hydroxycamptothecin Inhibits Peritendinous Adhesion via the Endoplasmic Reticulum Stress-Dependent Apoptosis
Abstract
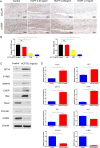
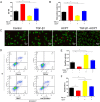
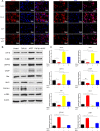
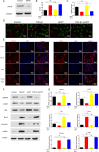
Traumatic peritendinous fibrosis is a worldwide clinical problem resulting in severe limb disability. Hydroxycamptothecin (HCPT) is an anti-neoplastic drug widely exploited in clinical practice. It has shown potential of anti-fibrosis in recent years. We previously demonstrated that HCPT inhibited the characterization of fibrosis in vitro. However, it is still unclear whether it ameliorates peritendinous adhesion in an in vivo animal tendon injury model. The underlying mechanism is also worth investigating. The present study aims to determine whether HCPT inhibits tendon adhesion and to explore the underlying mechanisms. In a rat tendon injury model, we observed that topical application of HCPT significantly attenuated peritendinous adhesion as revealed by the results of macroscopic observation, biomechanical, histological, immunohistochemical evaluation, western blot, and quantitative PCR (q-PCR) analyses. Furthermore, western blot and q-PCR analyses revealed that this phenomenon is correlated with HCPT activation of endoplasmic reticulum (ER) stress. In addition, in vitro studies show that HCPT significantly inhibits fibroblast proliferation and induces apoptosis by reducing the expression of extracellular matrix (ECM) proteins COL3A1 and α-smooth muscle actin (α-SMA). Finally, we employed small interfering RNA (siRNA) to target inositol requiring kinase 1 (IRE1) and activated transcription factor 6 (ATF-6) to verify that the effect of inhibitory fibrosis of HCPT disappears after knockdown of ATF-6 and IRE1, thereby suggesting that an anti-fibrotic effect of HCPT is mediated by the ER-dependent apoptotic pathway. In conclusion, our results indicate that HCPT inhibits peritendinous fibrosis through the ER-dependent apoptotic pathway and might serve as a potential solution to prevent traumatic peritendinous adhesion.
Keywords: ATF-6; IRE1; TGF-β1; hydroxycamptothecin; peritendinous fibrosis.
Figures


References
-
- Chern Y. J., Wong J. C. T., Cheng G. S. W., Yu A., Yin Y., Schaeffer D. F., et al. (2019). The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer. Cell Death Dis. 10 (7), 504. 10.1038/s41419-019-1687-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

