The Role of an Interventional Program for Improving Pharmacovigilance at a Pediatric Facility
- PMID: 31551794
- PMCID: PMC6746909
- DOI: 10.3389/fphar.2019.01004
The Role of an Interventional Program for Improving Pharmacovigilance at a Pediatric Facility
Abstract
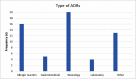
Background: The reporting rate of adverse drug reactions (ADRs) by healthcare professionals is low. ADR interventional programs may improve the reporting rate by the medical team. Our literature search revealed that only a few interventional studies among the pediatric population have been published. Objective: We aimed to create an interventional program in order to improve the reporting rate of ADRs during the interventional period compared to the control period, detect which drugs frequently lead to ADRs and determine the most serious ADRs. Design: A 3-month prospective intervention study compared with one year prior to the intervention (control period). ADR data was also collected for the year following the study period. Healthcare professionals were encouraged to report ADRs and an ADR reporting system was created for them. Setting: Pediatric Division at Shamir Medical Center (Assaf Harofeh), a tertiary care medical center. Results: The study population included 3,753 admitted patients with 1,323 prescriptions during the study period. During the period before the intervention was started, the ADR reporting rate was null. During the study period, 46 reports were collected: 46% from the general pediatric department, 26% from the pediatric neurology department, and 22% and 6% from the pediatric and neonatal intensive care units, respectively. Antiepileptic medications, IVIG, steroids and antibiotics were frequently reported to induce ADRs. Serious ADRs were also reported in 5 cases. One year of follow up after the intervention revealed a significant decline in the reporting rate. Conclusion: It is important to periodically encourage healthcare professionals to report any ADRs in order to increase knowledge about medication safety and prevent fatal harm.
Keywords: adverse drug reactions; intervention; pediatric; pharmacovigilance; reporting; sides effects.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials