Foxp3+ Regulatory T Cells in Bone and Hematopoietic Homeostasis
- PMID: 31551927
- PMCID: PMC6746882
- DOI: 10.3389/fendo.2019.00578
Foxp3+ Regulatory T Cells in Bone and Hematopoietic Homeostasis
Abstract
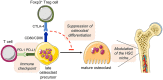
The bone represents surprisingly dynamic structures that are subject to constant remodeling by the concerted action of bone-forming osteoblasts and bone-resorbing osteoclasts - two cell subsets of distinct developmental origin that are key in maintaining skeletal integrity throughout life. In general, abnormal bone remodeling due to dysregulated bone resorption and formation is an early event in the manifestation of various human bone diseases, such as osteopetrosis/osteoporosis and arthritis. But bone remodeling is also closely interrelated with lympho-hematopoietic homeostasis, as the bone marrow niche is formed by solid and trabecular bone structures that provide a framework for the long-term maintenance and differentiation of HSCs (>blood lineage cells and osteoclasts) and MSCs (>osteoblasts). Numerous studies in mice and humans have implicated innate and adaptive immune cells in the dynamic regulation of bone homeostasis, but despite considerable clinical relevance, the exact mechanisms of such immuno-bone interplay have remained incompletely understood. This holds particularly true for CD4+ regulatory T (Treg) cells expressing the lineage specification factor Foxp3: Foxp3+ Treg cells have been shown to play an indispensable role in maintaining immune homeostasis, but may also exert critical non-immune functions, which includes the control of metabolic and regenerative processes, as well as the differentiation of HSCs and function of osteoclasts. Here, we summarize our current knowledge on the T cell/bone interplay, with a particular emphasis on our own efforts to dissect the role of Foxp3+ Treg cells in bone and hematopoietic homeostasis, employing experimental settings of gain- and loss-of-Treg cell function. These data make a strong case that Foxp3+ Treg cells impinge on lympho-hematopoiesis through indirect mechanisms, i.e., by acting on osteoclast development and function, which translates into changes in niche size. Furthermore, we propose that, besides disorders that involve inflammatory bone loss, the modulation of Foxp3+ Treg cell function in vivo may represent a suitable approach to reinstate bone homeostasis in non-autoimmune settings of aberrant bone remodeling.
Keywords: Foxp3+ Treg cells; bone disorders; bone microenvironment; lympho-hematopoiesis; osteoclasts.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

