Neonatal Exposure to Amoxicillin Alters Long-Term Immune Response Despite Transient Effects on Gut-Microbiota in Piglets
- PMID: 31552023
- PMCID: PMC6737505
- DOI: 10.3389/fimmu.2019.02059
Neonatal Exposure to Amoxicillin Alters Long-Term Immune Response Despite Transient Effects on Gut-Microbiota in Piglets
Abstract
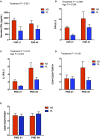
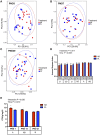
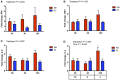
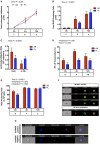
Antibiotic exposure during neonatal development may result in transient or persistent alterations of key microbes that are vital for normal development of local and systemic immunity, potentially impairing immune competence later in life. To further elucidate the relationship between antibiotic exposure and immune development, newborn pigs were exposed to a therapeutic pediatric dose (30 mg/kg/day) of amoxicillin (AB) or placebo (PL) from post-natal day (PND) 0-14. Subsequently, immune cell phenotype, microbial composition, and immune response to an intraperitoneal (IP) challenge with Salmonella enterica serovar Typhimurium were evaluated. AB exposure caused significant changes in fecal microbial composition on PND 3 (P = 0.025). This stemmed from a 2-fold increase in Enterobacteriaceae with live cecal coliforms on PND 7 indicating at 10-fold increase (P = 0.036). Alterations in microbial composition were transient, and successional patterns were normalizing by PND 14 (P = 0.693). Differences in PBMC (peripheral blood mononuclear cell) immune cell subtypes were detected, with the percentage of CD3+CD4+ T cells among the broader T cell population (CD3+CD4+/CD3+) being significantly higher (P = 0.031) in AB pigs and the numbers of CD4+CD45RA+ (naïve) T cells per liter of blood were lower on PND 21 in AB pigs (P = 0.036). Meanwhile, PBMCs from AB pigs produced significantly more IFNγ upon stimulation with a T-cell mitogen on PND 21 and 49 (P = 0.021). When AB pigs were challenged with heat-killed Salmonella (IP) on PND 49, IFNγ gene expression in peripheral blood was upregulated compared to those treated with PL (P = 0.043). Additionally, AB pigs showed stronger activation among neutrophils infiltrating the peritoneal cavity after in vivo immune challenge, based on higher levels of NF-κB nuclear translocation (P = 0.001). Overall, our results indicate that early life treatment with a therapeutically relevant dose of a commonly prescribed antibiotic has a programming effect on the immune system. Despite antibiotics only causing a transient disruption in gut-associated microbial communities, implications were long-term, with antibiotic treated pigs mounting an upregulated response to an immune challenge. This research adds to the growing body of evidence indicating adverse immune outcomes of early life antibiotic exposures.
Keywords: Salmonella; antibiotic; immune development; microbiota; pig model.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

