Cardiotrophin-Like Cytokine Factor 1 Exhibits a Myeloid-Biased Hematopoietic-Stimulating Function
- PMID: 31552057
- PMCID: PMC6746841
- DOI: 10.3389/fimmu.2019.02133
Cardiotrophin-Like Cytokine Factor 1 Exhibits a Myeloid-Biased Hematopoietic-Stimulating Function
Abstract
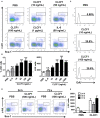
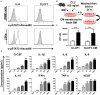
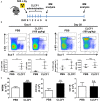
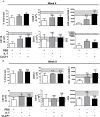
Cardiotrophin-like cytokine factor 1 (CLCF1) is secreted as a complex with the cytokine receptor-like factor 1 (CRLF1). Syndromes caused by mutations in the genes encoding CLCF1 or CRLF1 suggest an important role for CLCF1 in the development and regulation of the immune system. In mice, CLCF1 induces B-cell expansion, enhances humoral responses and triggers autoimmunity. Interestingly, inactivation of CRLF1, which impedes CLCF1 secretion, leads to a marked reduction in the number of bone marrow (BM) progenitor cells, while mice heterozygous for CLCF1 display a significant decrease in their circulating leukocytes. We therefore hypothesized that CLCF1 might be implicated in the regulation of hematopoiesis. To test this hypothesis, murine hematopoietic progenitor cells defined as Lin-Sca1+c-kit+ (LSK) were treated in vitro with ascending doses of CLCF1. The frequency and counts of LSK cells were significantly increased in the presence of CLCF1, which may be mediated by several CLCF1-induced soluble factors including IL-6, G-CSF, IL-1β, IL-10, and VEGF. CLCF1 administration to non-diseased C57BL/6 mice resulted in a pronounced increase in circulating myeloid cells, which was concomitant with augmented LSK and myeloid cell counts in the BM. Likewise, CLCF1 administration to mice following sub-lethal irradiation or congeneic BM transplantation (BMT) resulted in accelerated LSK recovery along with a sustained increase in BM-derived CD11b+ cells. Altogether, our observations establish an important and unforeseen role for CLCF1 in regulating hematopoiesis with a bias toward myeloid cell differentiation.
Keywords: LSK cells; bone marrow transplantation; cardiotrophin-like cytokine factor 1; interleukin-6; myelopoiesis.
Figures


References
-
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. (1993) 82:3515–23. - PubMed
-
- Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, et al. . Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. (1996) 93:407–11. 10.1073/pnas.93.1.407 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

