Part I of GANNET53: A European Multicenter Phase I/II Trial of the Hsp90 Inhibitor Ganetespib Combined With Weekly Paclitaxel in Women With High-Grade, Platinum-Resistant Epithelial Ovarian Cancer-A Study of the GANNET53 Consortium
- PMID: 31552170
- PMCID: PMC6746955
- DOI: 10.3389/fonc.2019.00832
Part I of GANNET53: A European Multicenter Phase I/II Trial of the Hsp90 Inhibitor Ganetespib Combined With Weekly Paclitaxel in Women With High-Grade, Platinum-Resistant Epithelial Ovarian Cancer-A Study of the GANNET53 Consortium
Abstract
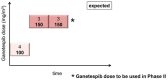
Background: Stabilized mutant p53 protein (mutp53) is a novel target in epithelial ovarian cancer. Due to aberrant conformation, mutp53 proteins depend on folding support by the Hsp90 chaperone. Hsp90 blockade induces degradation of mutp53, resulting in tumor cell cytotoxicity and increased sensitivity to chemotherapeutics. Preclinical synergy of the Hsp90 inhibitor ganetespib combined with paclitaxel provided the rationale for testing the combination in platinum-resistant ovarian cancer (PROC) patients in the GANNET53 trial (NCT02012192). Methods: Eligible patients had high-grade PROC with ≤ 4 prior lines of chemotherapy. Weekly paclitaxel (80 mg/m2) and increasing doses of ganetespib (100, 150 mg/m2) were given i.v. on days 1, 8, 15 in a 28 days cycle until disease progression or unacceptable toxicity. Endpoints were safety and determination of phase II dose. Dose limiting toxicity (DLT) was defined as grade 4 toxicity (with exceptions) occurring in cycles 1&2. Results: Ten patients (median age 59 years; range 43-70) were enrolled. No DLT occurred in cohort 1 (4 patients treated with paclitaxel + ganetespib 100 mg/m2), nor in cohorts 2 and 3 (6 patients treated with paclitaxel + ganetespib 150 mg/m2). The most common adverse event (AE) related to ganetespib was transient grade 1/2 diarrhea (n = 6). Related grade 1/2 AEs in >2 patients included QTc prolongation (n = 4), nausea (n = 3), anemia (n = 3), headache (n = 3), fatigue (n = 3), and dyspnoea (n = 3). Most frequently related grade 3/4 AEs were diarrhea (n = 3) and neutropenia (n = 2). There was 1 death on study due to hemorrhage from a duodenal ulcer. Three patients discontinued study treatment due to serious AEs (digestive hemorrhage n = 1, cardiac failure n = 1, abdominal pain and vomiting n = 1), 6 due to progressive disease, one due to investigator and patient decision. Two patients achieved a partial response (ORR 20%) and 4 patients a stable disease (disease control rate of 60%). Median PFS was 2.9 months (1.6 months in cohort 1 at 100 mg/m2 ganetespib, 5.1 months in cohorts 2+3 at 150 mg/m2 ganetespib). Conclusions: The combination of ganetespib 150 mg/m2 with paclitaxel 80 mg/m2 once weekly for 3 out of 4 weeks was generally well-tolerated with no DLTs, and therefore chosen for the randomized phase II trial.
Keywords: Hsp90 inhibitors; ganetespib; p53 mutation; platinum-resistance; recurrent ovarian carcinoma.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

