Vitamin D in Breastfed Infants: Systematic Review of Alternatives to Daily Supplementation
- PMID: 31552417
- PMCID: PMC7442322
- DOI: 10.1093/advances/nmz098
Vitamin D in Breastfed Infants: Systematic Review of Alternatives to Daily Supplementation
Abstract
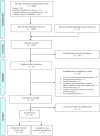
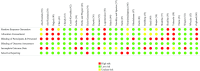
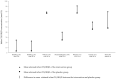
Daily oral vitamin D supplementation (400 IU) is recommended for breastfeeding infants (≤1 y). Recent studies have examined alternative approaches to preventing vitamin D deficiency in this population. This systematic review and meta-analysis aimed to estimate the effects of maternal postpartum (M-PP) or infant intermittent (I-INT) vitamin D supplementation on infant 25-hydroxyvitamin D [25(OH)D] concentrations in comparison to routine direct infant daily (I-D) oral supplementation (400 IU). MEDLINE, MEDLINE In-Process, Embase, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials were searched up to December 2018. Inclusion criteria consisted of published, peer-reviewed, vitamin D intervention trials involving lactating women and/or exclusively or partially breastfed term infants. Two reviewers independently extracted study characteristics (e.g., sample size, intervention dose, and duration and mode of administration) and related biochemical and clinical outcomes. Of 28 included trials, 5 randomized controlled trials were incorporated in meta-analyses examining infant 25(OH)D. Overall, M-PP supplementation resulted in modestly lower infant 25(OH)D compared with I-D supplementation (weighted mean difference = -8.1 nmol/L; 95% CI: -15.4, -0.9; I2 = 45%; P = 0.14; 3 trials), but the 2 most recent trials found M-PP to achieve similar infant 25(OH)D as I-D. Comparison of I-INT with I-D was confined to 2 trials with contradictory findings, and it was considered inappropriate for pooled analysis. Meta-analysis was therefore limited by a small number of eligible trials with variable quality of analytically derived 25(OH)D data and inconsistent reporting of safety outcomes, including effects on calcium homeostasis. Considering all 28 included trials, this systematic review highlights M-PP and I-INT regimens as plausible substitutes for routine daily infant vitamin D supplementation, but evidence remains too weak to support a policy update. Dose-ranging, adequately powered trials are required to establish the efficacy, safety, and feasibility of alternative strategies to prevent vitamin D deficiency in breastfeeding infants. This review was registered with PROSPERO as CRD42017069905.
Keywords: 25-hydroxyvitamin D; micronutrient supplementation; rickets; vitamin D; vitamin D supplementation.
Copyright © American Society for Nutrition 2019.
Figures

References
-
- Wagner CL, Greer FR.. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52. - PubMed
-
- Scientific Advisory Committee on Nutrition. Report on vitamin D and health. London: Public Health England; 2016.
-
- Scientific Advisory Committee on Nutrition. Update on vitamin D: position statement by the Scientific Advisory Committee on Nutrition. London: Public Health England; 2007.
-
- Infant Feeding Joint Working Group. Nutrition for healthy term infants: recommendations from birth to six months. [Internet][cited 2019 Mar 3]. Ottawa (Canada): Health Canada; Available from: https://www.canada.ca/en/health-canada/services/canada-food-guide/resour.... - PubMed

