Methodological description of clinical research data collection through electronic medical records in a center participating in an international multicenter study
- PMID: 31553359
- PMCID: PMC6748344
- DOI: 10.31744/einstein_journal/2019AE4791
Methodological description of clinical research data collection through electronic medical records in a center participating in an international multicenter study
Abstract
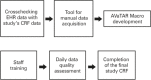
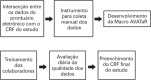
Data collection for clinical research can be difficult, and electronic health record systems can facilitate this process. The aim of this study was to describe and evaluate the secondary use of electronic health records in data collection for an observational clinical study. We used Cerner Millennium®, an electronic health record software, following these steps: (1) data crossing between the study's case report forms and the electronic health record; (2) development of a manual collection method for data not recorded in Cerner Millennium®; (3) development of a study interface for automatic data collection in the electronic health records; (4) employee training; (5) data quality assessment; and (6) filling out the electronic case report form at the end of the study. Three case report forms were consolidated into the electronic case report form at the end of the study. Researchers performed daily qualitative and quantitative analyses of the data. Data were collected from 94 patients. In the first case report form, 76.5% of variables were obtained electronically, in the second, 95.5%, and in the third, 100%. The daily quality assessment of the whole process showed complete and correct data, widespread employee compliance and minimal interference in their practice. The secondary use of electronic health records is safe and effective, reduces manual labor, and provides data reliability. Anesthetic care and data collection may be done by the same professional.
RESUMO: A coleta de dados para pesquisa clínica pode representar um desafio em que sistemas de registro eletrônico em saúde podem facilitar o processo. O objetivo deste estudo foi descrever e avaliar o uso secundário de registros eletrônicos em saúde na coleta de dados para um estudo clínico observacional. Usamos o Cerner Millennium®, software de registro eletrônico em saúde, de acordo com os seguintes passos: (1) cruzamento dos dados das fichas de coleta de dados do estudo e dos registros eletrônicos em saúde; (2) desenvolvimento de método para coleta manual de dados não registrados no Cerner Millennium®; (3) desenvolvimento de interface de estudo para a coleta automática de dados nos registros eletrônicos em saúde; (4) treinamento de colaboradores; (5) avaliação da qualidade dos dados; e (6) preenchimento da ficha eletrônica de coleta de dados no fim do estudo. Três fichas de coleta de dados foram consolidadas em uma ficha eletrônica de coleta de dados no fim do estudo. Os pesquisadores realizaram análise qualitativa e quantitativa de dados diariamente. Foram coletados dados de 94 pacientes. Na primeira ficha de coleta de dados, 76,5% das variáveis foram obtidas eletronicamente, na segunda, 95,5%, e na terceira, 100%. A avaliação diária de qualidade do processo como um todo revelou dados completos e corretos, ampla adesão dos colaboradores e mínima interferência na prática profissional. O uso secundário dos registros eletrônicos em saúde é seguro e efetivo, reduz o trabalho manual e produz dados confiáveis. O cuidado anestésico ao paciente e a coleta de dados podem ser realizados simultaneamente pelo mesmo professional.
Figures



References
-
- 1. Bruland P, McGilchrist M, Zapletal E, Acosta D, Proeve J, Askin S, et al. Common data elements for secondary use of electronic health record data for clinical trial execution and serious adverse event reporting. BMC Med Res Methodol. 2016;16(1):159. - PMC - PubMed
- Bruland P, McGilchrist M, Zapletal E, Acosta D, Proeve J, Askin S, et al. Common data elements for secondary use of electronic health record data for clinical trial execution and serious adverse event reporting. 159BMC Med Res Methodol. 2016;16(1) - PMC - PubMed
-
- 2. Coorevits P, Sundgren M, Klein GO, Bahr A, Claerhout B, Daniel C, et al. Electronic health records: new opportunities for clinical research. J Intern Med. 2013;274(6):547-60. Review. - PubMed
- Coorevits P, Sundgren M, Klein GO, Bahr A, Claerhout B, Daniel C, et al. Electronic health records: new opportunities for clinical research. J Intern Med. 2013;274(6):547–560. Review. - PubMed
-
- 4. van Schalkwyk JM, Lowes D, Frampton C, Merry AF. Does manual anaesthetic record capture remove clinically important data? Br J Anaesth. 2011;107(4):546-52. - PubMed
- van Schalkwyk JM, Lowes D, Frampton C, Merry AF. Does manual anaesthetic record capture remove clinically important data? Br J Anaesth. 2011;107(4):546–552. - PubMed
-
- 5. Queiroz VN, da Costa LG, Barbosa RP, Takaoka F, De Baerdemaeker L, Cesar DS, D’Orto UC, Galdi JR, Gottumukkala V, Cata JP, Hemmes SN, Hollman MW, Kalmar A, Moura LA, Mariano RM, Matot I, Mazzinari G, Mills GH, Posso IP, Teruya A, Vidal Melo MF, Sprung J, Weingarten TN, Treschan TA, Koopman S, Eidelman L, Chen LL, Lee JW, Ariño Irujo JJ, Tena B, Groeben H, Pelosi P, de Abreu MG, Schultz MJ, Serpa Neto A; AVATaR and PROVE Network investigators. International multicenter observational study on assessment of ventilatory management during general anaesthesia for robotic surgery and its effects on postoperative pulmonary complication (AVATaR): study protocol and statistical analysis plan. BMJ Open. 2018;8(8):e021643. - PMC - PubMed
- Queiroz VN, da Costa LG, Barbosa RP, Takaoka F, De Baerdemaeker L, Cesar DS, D’Orto UC, Galdi JR, Gottumukkala V, Cata JP, Hemmes SN, Hollman MW, Kalmar A, Moura LA, Mariano RM, Matot I, Mazzinari G, Mills GH, Posso IP, Teruya A, Vidal Melo MF, Sprung J, Weingarten TN, Treschan TA, Koopman S, Eidelman L, Chen LL, Lee JW, Ariño Irujo JJ, Tena B, Groeben H, Pelosi P, de Abreu MG, Schultz MJ, Serpa A, Neto, AVATaR and PROVE Network investigators International multicenter observational study on assessment of ventilatory management during general anaesthesia for robotic surgery and its effects on postoperative pulmonary complication (AVATaR): study protocol and statistical analysis plan. BMJ Open. 2018;8(8):e021643 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

