Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial
- PMID: 31553463
- PMCID: PMC6763995
- DOI: 10.1001/jamasurg.2019.3473
Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial
Abstract
Importance: Laparoscopic distal gastrectomy and neoadjuvant chemotherapy are increasingly used to treat locally advanced gastric cancer. However, the safety and efficacy of the laparoscopic procedure after neoadjuvant chemotherapy remain unclear.
Objective: To evaluate the short-term outcomes of patients with locally advanced gastric cancer who received either laparoscopic distal gastrectomy or open distal gastrectomy.
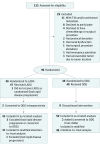
Design, setting, and participants: Between April 23, 2015, and November 16, 2017, a phase 2, open-label, noninferiority randomized clinical trial was conducted at the Gastrointestinal Cancer Center of Peking University Cancer Hospital and Institute in Beijing, China. Patients (n = 96) between 18 and 80 years of age with locally advanced gastric cancer (cT2-4aN+M0) who were receiving neoadjuvant chemotherapy were enrolled and randomized. An as-treated population and a modified intention-to-treat (mITT) population were defined for the data analysis.
Interventions: Patients were randomized to undergo either laparoscopy-assisted distal gastrectomy (LADG) with D2 lymphadenectomy or open distal gastrectomy (ODG) with D2 lymphadenectomy.
Main outcomes and measures: The primary end point was 3-year recurrence-free survival rate. Secondary end points were surgical radicality, 30-day postoperative morbidity and mortality, 2-week postoperative recovery indexes, and adjuvant chemotherapy completion status.
Results: In total, 95 patients were eligible for as-treated analyses (LADG: 45, of whom 13 were female [29%], with a median [interquartile range (IQR)] age of 59 [52-65] years; ODG: 50, of whom 16 were female [32%], with a median [IQR] age of 61 [55-64] years) and mITT analyses (LADG: 47, of whom 14 were female [30%], with a median [IQR] age of 59 [52-65] years; ODG: 48, of whom 15 were female [31%], with a median [IQR] age of 61 [55-64] years). In the as-treated analyses, the LADG group had a significantly lower postoperative complication rate than the ODG group (20% vs 46%; P = .007). The postoperative visual analog scale score for pain was 1.2 units lower on postoperative day 2 only in the LADG group (95% CI, -2.1 to -0.3; P = .008). Patients in the LADG group had better adjuvant chemotherapy completion (adjusted odds ratio, 4.39; 95% CI, 1.63-11.80; P = .003) and were less likely to terminate adjuvant chemotherapy because of adverse effects (10 [22%] vs 21 [42%]; P = .04). The mITT analyses showed similar results to as-treated analyses.
Conclusions and relevance: This trial found that LADG appears to offer the benefits of better postoperative safety and adjuvant chemotherapy tolerance compared with ODG for patients with locally advanced gastric cancer who received neoadjuvant chemotherapy.
Trial registration: ClinicalTrials.gov identifier: NCT02404753.
Conflict of interest statement
Figures
Comment in
-
Laparoscopic Resection After Neoadjuvant Chemotherapy for Distal Gastric Tumors: Safe, but Is It Better?JAMA Surg. 2019 Dec 1;154(12):1101-1102. doi: 10.1001/jamasurg.2019.3474. JAMA Surg. 2019. PMID: 31553415 No abstract available.
-
Laparoscopic Gastrectomy After Neoadjuvant Chemotherapy: Still Far From Solid Conclusions.JAMA Surg. 2020 May 1;155(5):449-450. doi: 10.1001/jamasurg.2019.5943. JAMA Surg. 2020. PMID: 32074265 No abstract available.
-
Laparoscopic Gastrectomy After Neoadjuvant Chemotherapy-Reply.JAMA Surg. 2020 May 1;155(5):450-451. doi: 10.1001/jamasurg.2019.5946. JAMA Surg. 2020. PMID: 32074280 No abstract available.
-
Oncological outcomes of sequential laparoscopic gastrectomy after treatment with camrelizumab combined with nab-paclitaxel plus S-1 for gastric cancer with serosal invasion.Front Immunol. 2024 Jan 25;15:1322152. doi: 10.3389/fimmu.2024.1322152. eCollection 2024. Front Immunol. 2024. PMID: 38333217 Free PMC article.
References
-
- Inaki N, Etoh T, Ohyama T, et al. A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg. 2015;39(11):2734-2741. doi: 10.1007/s00268-015-3160-z - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous