Molecular Dynamics Study of the Hybridization between RNA and Modified Oligonucleotides
- PMID: 31553600
- PMCID: PMC6889957
- DOI: 10.1021/acs.jctc.9b00519
Molecular Dynamics Study of the Hybridization between RNA and Modified Oligonucleotides
Abstract
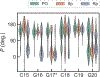
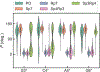
MicroRNAs (miRNAs) are attractive drug candidates for many diseases as they can modulate the expression of gene networks. Recently, we discovered that DNAs targeting microRNA-22-3p (miR-22-3p) hold the potential for treating obesity and related metabolic disorders (type 2 diabetes mellitus, hyperlipidemia, and nonalcoholic fatty liver disease (NAFLD)) by turning fat-storing white adipocytes into fat-burning adipocytes. In this work, we explored the effects of chemical modifications, including phosphorothioate (PS), locked nucleic acid (LNA), and peptide nucleic acid (PNA), on the structure and energy of DNA analogs by using molecular dynamics (MD) simulations. To achieve a reliable prediction of the hybridization free energy, the AMOEBA polarizable force field and the free energy perturbation technique were employed. The calculated hybridization free energies are generally compatible with previous experiments. For LNA and PNA, the enhanced duplex stability can be explained by the preorganization mechanism, i.e., the single strands adopt stable helical structures similar to those in the duplex. For PS, the S and R isomers (Sp and Rp) have preferences for C2'-endo and C3'-endo sugar puckering conformations, respectively, and therefore Sp is less stable than Rp in DNA/RNA hybrids. In addition, the solvation penalty of Rp accounts for its destabilization effect. PS-LNA is similar to LNA as the sugar puckering is dominated by the locked sugar ring. This work demonstrated that MD simulations with polarizable force fields are useful for the understanding and design of modified nucleic acids.
Conflict of interest statement
The authors declare the following conflict of interest. MT is the Founder and President of AptamiR Therapeutics, Inc. MT is the inventor of patents that are assigned to AptamiR Therapeutics, Inc.
Figures



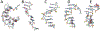


Similar articles
-
Interplay of LNA and 2'-O-methyl RNA in the structure and thermodynamics of RNA hybrid systems: a molecular dynamics study using the revised AMBER force field and comparison with experimental results.J Phys Chem B. 2014 Dec 11;118(49):14177-87. doi: 10.1021/jp506703g. Epub 2014 Sep 30. J Phys Chem B. 2014. PMID: 25268896
-
Structures, dynamics, and stabilities of fully modified locked nucleic acid (β-D-LNA and α-L-LNA) duplexes in comparison to pure DNA and RNA duplexes.J Phys Chem B. 2013 May 9;117(18):5556-64. doi: 10.1021/jp4016068. Epub 2013 Apr 25. J Phys Chem B. 2013. PMID: 23617391
-
Insights into structure, dynamics and hydration of locked nucleic acid (LNA) strand-based duplexes from molecular dynamics simulations.Nucleic Acids Res. 2008 Mar;36(5):1508-16. doi: 10.1093/nar/gkm1182. Epub 2008 Jan 18. Nucleic Acids Res. 2008. PMID: 18203740 Free PMC article.
-
Locked nucleic acid oligonucleotides: the next generation of antisense agents?BioDrugs. 2007;21(4):235-43. doi: 10.2165/00063030-200721040-00004. BioDrugs. 2007. PMID: 17628121 Review.
-
Influence of Sugar Modifications on the Nucleoside Conformation and Oligonucleotide Stability: A Critical Review.Chem Rec. 2022 Dec;22(12):e202200174. doi: 10.1002/tcr.202200174. Epub 2022 Sep 1. Chem Rec. 2022. PMID: 36048010 Review.
Cited by
-
Thermostability, Tunability, and Tenacity of RNA as Rubbery Anionic Polymeric Materials in Nanotechnology and Nanomedicine-Specific Cancer Targeting with Undetectable Toxicity.Chem Rev. 2021 Jul 14;121(13):7398-7467. doi: 10.1021/acs.chemrev.1c00009. Epub 2021 May 26. Chem Rev. 2021. PMID: 34038115 Free PMC article. Review.
-
Structural dynamics of therapeutic nucleic acids with phosphorothioate backbone modifications.NAR Genom Bioinform. 2024 May 25;6(2):lqae058. doi: 10.1093/nargab/lqae058. eCollection 2024 Jun. NAR Genom Bioinform. 2024. PMID: 38800826 Free PMC article.
-
Molecular Dynamics Simulations of Protein RNA Complexes by Using an Advanced Electrostatic Model.J Phys Chem B. 2022 Sep 29;126(38):7343-7353. doi: 10.1021/acs.jpcb.2c05278. Epub 2022 Sep 15. J Phys Chem B. 2022. PMID: 36107618 Free PMC article.
-
Computational study on the binding of Mango-II RNA aptamer and fluorogen using the polarizable force field AMOEBA.Front Mol Biosci. 2022 Sep 2;9:946708. doi: 10.3389/fmolb.2022.946708. eCollection 2022. Front Mol Biosci. 2022. PMID: 36120549 Free PMC article.
-
Detection of miR-155 Using Peptide Nucleic Acid at Physiological-like Conditions by Surface Plasmon Resonance and Bio-Field Effect Transistor.Biosensors (Basel). 2024 Feb 1;14(2):79. doi: 10.3390/bios14020079. Biosensors (Basel). 2024. PMID: 38391998 Free PMC article.
References
-
- Smith CIE; Zain R, Therapeutic Oligonucleotides: State of the Art. Annu Rev Pharmacol 2019, 59 (1), 605–630. - PubMed
-
- Bartel DP, MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116 (2), 281–297. - PubMed
-
- Rupaimoole R.; Slack FJ, MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature Reviews Drug Discovery 2017, 16, 203. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
