Body side-specific changes in sensorimotor processing of movement feedback in a walking insect
- PMID: 31553676
- PMCID: PMC6879953
- DOI: 10.1152/jn.00436.2019
Body side-specific changes in sensorimotor processing of movement feedback in a walking insect
Abstract
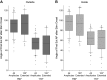
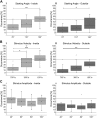
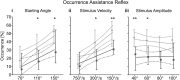
Feedback from load and movement sensors can modify timing and magnitude of the motor output in the stepping stick insect. One source of feedback is stretch reception by the femoral chordotonal organ (fCO), which encodes such parameters as the femorotibial (FTi) joint angle, the angular velocity, and its acceleration. Stimulation of the fCO causes a postural resistance reflex, during quiescence, and can elicit the opposite, so-called active reaction (AR), which assists ongoing flexion during active movements. In the present study, we investigated the role of fCO feedback for the difference in likelihood of generating ARs on the inside vs. the outside during curve stepping. We analyzed the effects of fCO stimulation on the motor output to the FTi and the neighboring coxa-trochanter and thorax-coxa joints of the middle leg. In inside and outside turns, the probability for ARs increases with increasing starting angle and decreasing stimulus velocity; furthermore, it is independent of the total angular excursion. However, the transition between stance and swing motor activity always occurs after a specific angular excursion, independent of the turning direction. Feedback from the fCO also has an excitatory influence on levator trochanteris motoneurons (MNs) during inside and outside turns, whereas the same feedback affects protractor coxae MNs only during outside steps. Our results suggest joint- and body side-dependent processing of fCO feedback. A shift in gain may be responsible for different AR probabilities between inside and outside turning, whereas the general control mechanism for ARs is unchanged.NEW & NOTEWORTHY We show that parameters of movement feedback from the tibia in an insect during curve walking are processed in a body side-specific manner, and how. From our results it is highly conceivable that the difference in motor response to the feedback supports the body side-specific leg kinematics during turning. Future studies will need to determine the source for the inputs that determine the local changes in sensory-motor processing.
Keywords: electrophysiology; motor control; reflex; sensorimotor; stick insect.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Bässler U. Neural Basis of Elementary Behavior in Stick Insects. Berlin: Springer, 1983.
-
- Bässler U. Afferent control of walking movements in the stick insect Cuniculina impigra. I. Decerebrated animals on a treadband. J Comp Physiol A 158: 345–349, 1986a. doi: 10.1007/BF00603618. - DOI
-
- Bässler U. Afferent control of walking movements in the stick insect Cuniculina impigra. II. Reflex reversal and the release of the swing phase in the restrained foreleg. J Comp Physiol 158: 351–362, 1986b. doi: 10.1007/BF00603619. - DOI
-
- Bässler U. Functional principles of pattern generation for walking movements of stick insect forelegs: the role of the femoral chordotonal organ afferences. J Exp Biol 136: 125–147, 1988.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

