Expedient Synthesis of Core Disaccharide Building Blocks from Natural Polysaccharides for Heparan Sulfate Oligosaccharide Assembly
- PMID: 31553820
- PMCID: PMC6901730
- DOI: 10.1002/anie.201908805
Expedient Synthesis of Core Disaccharide Building Blocks from Natural Polysaccharides for Heparan Sulfate Oligosaccharide Assembly
Abstract
The complex sulfation motifs of heparan sulfate glycosaminoglycans (HS GAGs) play critical roles in many important biological processes. However, an understanding of their specific functions has been hampered by an inability to synthesize large numbers of diverse, yet defined, HS structures. Herein, we describe a new approach to access the four core disaccharides required for HS/heparin oligosaccharide assembly from natural polysaccharides. The use of disaccharides rather than monosaccharides as minimal precursors greatly accelerates the synthesis of HS GAGs, providing key disaccharide and tetrasaccharide intermediates in about half the number of steps compared to traditional strategies. Rapid access to such versatile intermediates will enable the generation of comprehensive libraries of sulfated oligosaccharides for unlocking the "sulfation code" and understanding the roles of specific GAG structures in physiology and disease.
Keywords: carbohydrates; glycosaminoglycans; heparan sulfate; oligosaccharides; synthesis design.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
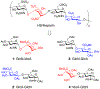


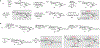
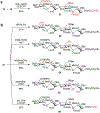

References
-
- Petitou M, van Boeckel CAA, Angew. Chem. Int. Ed 2004, 43, 3118–3133. - PubMed
- Liu J, Thorp SC, Med. Res. Rev 2002, 22, 1–25. - PubMed
- Corti F, Wang Y, Rhodes JM, Atri D, Archer-Hartmann S, Zhang J, Zhuang ZW, Chen D, Wang T, Wang Z, Azadi P, Simons M, Nat. Commun 2019, 10 (1), 1562. - PMC - PubMed
- Zong C, Venot A, Li X, Lu W, Xiao W, Wilkes J-SL, Salanga CL, Handel TM, Wang L, Wolfert MA, Boons G-J, J. Am. Chem. Soc 2017, 139 (28), 9534–9543. - PMC - PubMed
- Vlodavsky I, Gross-Cohen M, Weissmann M, Ilan N, Sanderson RD, Trends. Biochem. Sci 2018, 43 (1), 18–31. - PMC - PubMed
- Kaltenbach DD, Jaishankar D, Hao M, Beer JC, Volin MV, Desai UR, Tiwari V, Front Pharmacol. 2018, 9, 1315. - PMC - PubMed
- Lortat-Jacob H, Curr. Opin. Struct. Biol 2009, 19 (5), 543–548. - PubMed
- Collins LE, Troeberg L, J. Leukoc. Biol 2019, 105 (1), 81–92. - PubMed
- Zhang P, Lu H, Peixoto RT, Pines MK, Ge Y, Oku S, Siddiqui TJ, Xie Y, Wu W, Archer-Hartmann S, Yoshida K, Tanaka KF, Aricescu AR, Azadi P, Gordon MD, Sabatini BL, Wong ROL, Craig AM, Cell, 2018, 174 (6), 1450–1464. - PMC - PubMed
-
- Shi X, Zaia J, J. Biol. Chem 2009, 284, 11806–11814. - PMC - PubMed
- Feyzi E, Saldeen T, Larsson E, Lindahl U, Salmivirta M, J. Biol. Chem 1998, 273, 13395–13398. - PubMed
- Brickman YG, Ford MD, Gallagher JT, Nurcombe V, Bartlett PF, Turnbull JE, J. Biol. Chem 1998, 273, 4350–4359. - PubMed
- Lindahl U, Kjellén L, J. Intern. Med 2013, 273, 555–571. - PubMed
- Nadanaka S,Kitagawa H, J. Biochem 2008, 144, 7–14. - PubMed
-
- Dey S, Wong C-H, Chem. Sci 2018, 9, 6685–6691. - PMC - PubMed
- Hu Y-P, Lin S-Y, Huang C-Y, Zulueta MML, Liu J-Y, Chang W, Hung S-C, Nat. Chem 2011, 3, 557–563. - PubMed
- Orgueira HA, Bartolozzi A, Schell P; Litjens REJN, Palmacci ER, Seeberger PH, Chem. Eur. J 2003, 9, 140–169. - PubMed
- Yang B, Yoshida K, Yin Z, Dai H, Kavunja H, El-Dakdouki MH, Sungsuwan S, Dulaney SB, Huang X, Angew. Chem. Int. Ed 2012, 51, 10185–10189. - PMC - PubMed
- Zong C, Huang R, Condac E, Chiu Y, Xiao W, Li X, Lu W, Ishihara M, Wang S, Ramiah A, Stickney M, Azadi P, Amster J, Moremen KW, Wang L, Sharp JS, Boons G-J, J. Am. Chem. Soc 2016, 138, 13059–13067. - PMC - PubMed
- Codée JDC, Stubba B, Schiattarella M, Overkleeft HS, van Boeckel CAA, van Boom JH, van der Marel GA, J. Am. Chem. Soc 2005, 127, 3767–3773. - PubMed
- Hansen SU, Miller GJ, Cole C, Rushton G, Avizienyte E, Jayson GC; Gardiner JM, Nat. Commun 2013, 4, 2016. - PMC - PubMed
- Ojeda R, de Paz J-L, Martín-Lomas M, Chem. Commun 2003, 19, 2486–2487. - PubMed
- Xu Y, Masuko S, Takieddin M, Xu H, Liu R, Jing J, Mousa SA, Linhardt RJ,; J. Liu, Science, 2011, 334, 498–501. - PMC - PubMed
- Chen Y, Li Y, Yu H, Sugiarto G, Thon V, Hwang J, Ding L, Hie L, Chen X, Angew. Chem. Int. Ed 2013, 52, 11852–11856. - PMC - PubMed
- Wu B, Wei N, Thon V, Wei M, Yu Z, Xu Y, Chen X, Liu J, Wang PG, Li T, Org. Biomol. Chem 2015, 13, 5098–5101. - PMC - PubMed
- Mende M, Bednarek C, Wawryszyn M, Sauter P, Biskup MB, Schepers U, Bräse S, Chem. Rev 2016, 116, 8193–8255. - PubMed
- Hansen SU, Baráth M, Salameh BAB, Pritchard RG, Stimpson WT, Gardiner JM, Jayson GC, Org. Lett 2009, 11, 4528–4531. - PubMed
- Hansen SU, Dalton CE, Baráth M, Kwan G, Raftery J, Jayson GC, Miller GJ, Gardiner JM, J. Org. Chem 2015, 80, 3777–3789. - PubMed
- Hu Y-P, Zhong Y-Q, Chen Z-G, Chen C-Y, Shi Z, Zulueta MML, Ku C-C, Lee P-Y, Wang C-C, Hung S-C, J. Am. Chem. Soc 2012, 134, 20722–20727. - PubMed
-
- Liu X, St. Ange K, Fareed J, hoppensteadt D, Jeske W, Kouta A, Chi L, Jin C, Yao Y, Linhardt RJ, Clin. Appl. Thromb. Hemost 2017, 23, 542–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

