hESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson's Disease
- PMID: 31553914
- PMCID: PMC6899556
- DOI: 10.1016/j.celrep.2019.08.058
hESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson's Disease
Abstract
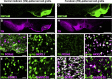
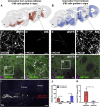
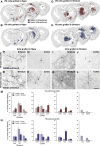
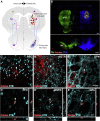
Cell replacement is currently being explored as a therapeutic approach for neurodegenerative disease. Using stem cells as a source, transplantable progenitors can now be generated under conditions compliant with clinical application in patients. In this study, we elucidate factors controlling target-appropriate innervation and circuitry integration of human embryonic stem cell (hESC)-derived grafts after transplantation to the adult brain. We show that cell-intrinsic factors determine graft-derived axonal innervation, whereas synaptic inputs from host neurons primarily reflect the graft location. Furthermore, we provide evidence that hESC-derived dopaminergic grafts transplanted in a long-term preclinical rat model of Parkinson's disease (PD) receive synaptic input from subtypes of host cortical, striatal, and pallidal neurons that are known to regulate the function of endogenous nigral dopamine neurons. This refined understanding of how graft neurons integrate with host circuitry will be important for the design of clinical stem-cell-based replacement therapies for PD, as well as for other neurodegenerative diseases.
Keywords: Parkinson’s disease; brain repair; cell replacement therapy; cell transplantation; grafting; monosynaptic tracing; stem cells.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
M.P. is the owner of Parmar Cells AB and co-inventor of the U.S. patent application 15/093,927 owned by Biolamina AB and EP17181588 owned by Miltenyi Biotech.
Figures



References
-
- Adams J.C. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J. Histochem. Cytochem. 1992;40:1457–1463. - PubMed
-
- Bachoud-Lévi A.C. From open to large-scale randomized cell transplantation trials in Huntington’s disease: Lessons from the multicentric intracerebral grafting in Huntington’s disease trial (MIG-HD) and previous pilot studies. Prog. Brain Res. 2017;230:227–261. - PubMed
-
- Barker R.A., Barrett J., Mason S.L., Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

