Mid-cervical interneuron networks following high cervical spinal cord injury
- PMID: 31553921
- PMCID: PMC6864252
- DOI: 10.1016/j.resp.2019.103305
Mid-cervical interneuron networks following high cervical spinal cord injury
Abstract
Spinal interneuron (IN) networks can facilitate respiratory motor recovery after spinal cord injury (SCI). We hypothesized that excitatory synaptic connectivity between INs located immediately caudal to unilateral cervical SCI would be most prevalent in a contra- to ipsilateral direction. Adult rats were studied following chronic C2 spinal cord hemisection (C2Hx) injury. Rats were anesthetized and ventilated and a multi-electrode array was used to simultaneously record INs on both sides of the C4-5 spinal cord. The temporal firing relationship between IN pairs was evaluated using cross-correlation with directionality of synaptic connections inferred based on electrode location. During baseline recordings, the majority of detectable excitatory IN connections occurred in a contra- to- ipsilateral direction. However, acute respiratory stimulation with hypoxia abolished this directionality, while simultaneously increasing the detectable inhibitory connections within the ipsilateral cord. We conclude that propriospinal networks caudal to SCI can display a contralateral-to-ipsilateral directionality of synaptic connections and that these connections are modulated by acute exposure to hypoxia.
Keywords: Cervical interneurons; Connectivity; Plasticity; Spinal cord injury.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing financial interests.
Figures
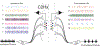
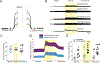

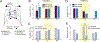
Similar articles
-
Intermittent Hypoxia Enhances Functional Connectivity of Midcervical Spinal Interneurons.J Neurosci. 2017 Aug 30;37(35):8349-8362. doi: 10.1523/JNEUROSCI.0992-17.2017. Epub 2017 Jul 27. J Neurosci. 2017. PMID: 28751456 Free PMC article.
-
Phrenic motor outputs in response to bronchopulmonary C-fibre activation following chronic cervical spinal cord injury.J Physiol. 2016 Oct 15;594(20):6009-6024. doi: 10.1113/JP272287. Epub 2016 Jun 3. J Physiol. 2016. PMID: 27106483 Free PMC article.
-
High-frequency epidural stimulation across the respiratory cycle evokes phrenic short-term potentiation after incomplete cervical spinal cord injury.J Neurophysiol. 2017 Oct 1;118(4):2344-2357. doi: 10.1152/jn.00913.2016. Epub 2017 Jun 14. J Neurophysiol. 2017. PMID: 28615341 Free PMC article.
-
Pre-phrenic interneurons: Characterization and role in phrenic pattern formation and respiratory recovery following spinal cord injury.Respir Physiol Neurobiol. 2019 Jul;265:24-31. doi: 10.1016/j.resp.2018.09.005. Epub 2018 Oct 10. Respir Physiol Neurobiol. 2019. PMID: 30315961 Review.
-
Serotonergic innervation of respiratory motor nuclei after cervical spinal injury: Impact of intermittent hypoxia.Exp Neurol. 2021 Apr;338:113609. doi: 10.1016/j.expneurol.2021.113609. Epub 2021 Jan 15. Exp Neurol. 2021. PMID: 33460645 Free PMC article. Review.
Cited by
-
Mitochondrial adaptations to inactivity in diaphragm muscle fibers.J Appl Physiol (1985). 2022 Jul 1;133(1):191-204. doi: 10.1152/japplphysiol.00090.2022. Epub 2022 Jun 9. J Appl Physiol (1985). 2022. PMID: 35678745 Free PMC article.
-
Closed-Loop, Cervical, Epidural Stimulation Elicits Respiratory Neuroplasticity after Spinal Cord Injury in Freely Behaving Rats.eNeuro. 2022 Feb 9;9(1):ENEURO.0426-21.2021. doi: 10.1523/ENEURO.0426-21.2021. Print 2022 Jan-Feb. eNeuro. 2022. PMID: 35058311 Free PMC article.
-
Contrasting Experimental Rodent Aftercare With Human Clinical Treatment for Cervical Spinal Cord Injury: Bridging the Translational "Valley of Death".J Neurotrauma. 2023 Dec;40(23-24):2469-2486. doi: 10.1089/neu.2023.0314. Epub 2023 Nov 10. J Neurotrauma. 2023. PMID: 37772694 Free PMC article. Review.
-
Targeted activation of spinal respiratory neural circuits.Exp Neurol. 2020 Jun;328:113256. doi: 10.1016/j.expneurol.2020.113256. Epub 2020 Feb 19. Exp Neurol. 2020. PMID: 32087253 Free PMC article. Review.
-
Ultrasound monitoring respiratory muscle rehabilitation training can promote the recovery of diaphragmatic function in traumatic spinal cord injury (TSCI) patients.Eur J Med Res. 2025 Aug 11;30(1):733. doi: 10.1186/s40001-025-03023-2. Eur J Med Res. 2025. PMID: 40790540 Free PMC article. Clinical Trial.
References
-
- Aertsen AM, Gerstein GL, 1985. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res. 340, 341–354. - PubMed
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G, 1989. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J. Neurophysiol 61, 900–917. - PubMed
-
- Bach KB, Kinkead R, Mitchell GS, 1999. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and alpha2-adrenoreceptor antagonism. Brain Res. 817, 25–33. - PubMed
-
- Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R, 2004. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J. Appl. Physiol. (1985) 96, 2057–2072. - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci 7, 48–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

