Leptin Promotes Expression of EMT-Related Transcription Factors and Invasion in a Src and FAK-Dependent Pathway in MCF10A Mammary Epithelial Cells
- PMID: 31554180
- PMCID: PMC6829404
- DOI: 10.3390/cells8101133
Leptin Promotes Expression of EMT-Related Transcription Factors and Invasion in a Src and FAK-Dependent Pathway in MCF10A Mammary Epithelial Cells
Abstract
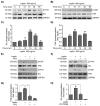
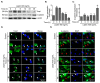
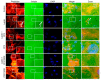
Leptin is one of the main adipokines secreted in breast tissue. Leptin promotes epithelial-mesenchymal transition (EMT), cell migration and invasion in epithelial breast cells, leading to tumor progression. Although, the molecular mechanisms that underlie these events are not fully understood, the activation of different signaling pathways appears to be essential. In this sense, the effects of leptin on the activation of kinases like Src and FAK, which regulate signaling pathways that activate the EMT program, are not completely described. Therefore, we investigated the involvement of these kinases using an in vitro model for leptin-induced EMT process in the non-tumorigenic MCF10A cell line. To this end, MCF10A cells were stimulated with leptin, and Src and FAK activation was assessed. Specific events occurring during EMT were also evaluated in the presence or absence of the kinases' chemical inhibitors PP2 and PF-573228. For instance, we tested the expression and subcellular localization of the EMT-related transcription factors Twist and β-catenin, by western blot and immunofluorescence. We also evaluated the secretion and activation of matrix metalloproteases (MMP-2 and MMP-9) by gelatin zymography. Invasiveness properties of leptin-stimulated cells were determined by invadopodia formation assays, and by the Transwell chamber method. Our results showed that leptin promotes EMT through Src and FAK activation, which leads to the secretion and activation of MMP-2 and MMP-9, invadopodia formation and cell invasion in MCF10A cells. In conclusion, our data suggest that leptin promotes an increase in the expression levels of Twist and β-catenin, the secretion of MMP-2, MMP-9, the invadopodia formation and invasion in MCF10A cells in a Src and FAK-dependent manner.
Keywords: EMT; FAK; Src; invasion; leptin; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The Leptin induced Hic-5 expression and actin puncta formation by the FAK/Src-dependent pathway in MCF10A mammary epithelial cells.Biomedica. 2019 Sep 1;39(3):547-560. doi: 10.7705/biomedica.4313. Biomedica. 2019. PMID: 31584768 Free PMC article. English, Spanish.
-
Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network.Int J Biochem Cell Biol. 2016 Feb;71:62-71. doi: 10.1016/j.biocel.2015.12.004. Epub 2015 Dec 13. Int J Biochem Cell Biol. 2016. PMID: 26693891
-
Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways.Oncotarget. 2015 Sep 22;6(28):25755-69. doi: 10.18632/oncotarget.4697. Oncotarget. 2015. PMID: 26342198 Free PMC article.
-
New Actors Driving the Epithelial-Mesenchymal Transition in Cancer: The Role of Leptin.Biomolecules. 2020 Dec 15;10(12):1676. doi: 10.3390/biom10121676. Biomolecules. 2020. PMID: 33334030 Free PMC article. Review.
-
Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition.Cell Commun Signal. 2021 Jun 30;19(1):67. doi: 10.1186/s12964-021-00750-x. Cell Commun Signal. 2021. PMID: 34193161 Free PMC article. Review.
Cited by
-
Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned so Far.Cancers (Basel). 2020 Jun 2;12(6):1448. doi: 10.3390/cancers12061448. Cancers (Basel). 2020. PMID: 32498343 Free PMC article. Review.
-
A Novel Model of Cancer Drug Resistance: Oncosomal Release of Cytotoxic and Antibody-Based Drugs.Biology (Basel). 2020 Mar 5;9(3):47. doi: 10.3390/biology9030047. Biology (Basel). 2020. PMID: 32150875 Free PMC article. Review.
-
Zinc transporter SLC39A13/ZIP13 facilitates the metastasis of human ovarian cancer cells via activating Src/FAK signaling pathway.J Exp Clin Cancer Res. 2021 Jun 21;40(1):199. doi: 10.1186/s13046-021-01999-3. J Exp Clin Cancer Res. 2021. PMID: 34154618 Free PMC article.
-
ZIP11 Regulates Nuclear Zinc Homeostasis in HeLa Cells and Is Required for Proliferation and Establishment of the Carcinogenic Phenotype.Front Cell Dev Biol. 2022 Jul 11;10:895433. doi: 10.3389/fcell.2022.895433. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35898402 Free PMC article.
-
Functional effect of indole-3 carbinol in the viability and invasive properties of cultured cancer cells.Biochem Biophys Rep. 2023 Jun 1;35:101492. doi: 10.1016/j.bbrep.2023.101492. eCollection 2023 Sep. Biochem Biophys Rep. 2023. PMID: 37304131 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

