Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1)
- PMID: 31554181
- PMCID: PMC6835877
- DOI: 10.3390/nu11102283
Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1)
Abstract
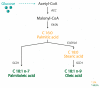
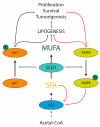
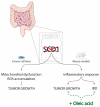
The consumption of an olive oil rich diet has been associated with the diminished incidence of cardiovascular disease and cancer. Several studies have attributed these beneficial effects to oleic acid (C18 n-9), the predominant fatty acid principal component of olive oil. Oleic acid is not an essential fatty acid since it can be endogenously synthesized in humans. Stearoyl-CoA desaturase 1 (SCD1) is the enzyme responsible for oleic acid production and, more generally, for the synthesis of monounsaturated fatty acids (MUFA). The saturated to monounsaturated fatty acid ratio affects the regulation of cell growth and differentiation, and alteration in this ratio has been implicated in a variety of diseases, such as liver dysfunction and intestinal inflammation. In this review, we discuss our current understanding of the impact of gene-nutrient interactions in liver and gut diseases, by taking advantage of the role of SCD1 and its product oleic acid in the modulation of different hepatic and intestinal metabolic pathways.
Keywords: MUFA; gut; liver; oleic acid; olive oil; stearoyl-CoA desaturase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate.Gastroenterology. 2018 Nov;155(5):1524-1538.e9. doi: 10.1053/j.gastro.2018.07.032. Epub 2018 Jul 29. Gastroenterology. 2018. PMID: 30063922
-
Vitamin A deficiency increases the oleic acid (C18:1) levels in the kidney of high fructose diet-fed rats.Indian J Med Res. 2019 Dec;150(6):620-629. doi: 10.4103/ijmr.IJMR_1574_17. Indian J Med Res. 2019. PMID: 32048626 Free PMC article.
-
Vitamin A deficiency induces endoplasmic reticulum stress and apoptosis in pancreatic islet cells: Implications of stearoyl-CoA desaturase 1-mediated oleic acid synthesis.Exp Cell Res. 2018 Mar 1;364(1):104-112. doi: 10.1016/j.yexcr.2018.01.040. Epub 2018 Feb 1. Exp Cell Res. 2018. PMID: 29409806
-
Regulation of stearoyl-CoA desaturases and role in metabolism.Prog Lipid Res. 2004 Mar;43(2):91-104. doi: 10.1016/s0163-7827(03)00039-0. Prog Lipid Res. 2004. PMID: 14654089 Review.
-
[Stearoyl-CoA desaturase in the control of metabolic homeostasis].Postepy Biochem. 2012;58(2):166-74. Postepy Biochem. 2012. PMID: 23214140 Review. Polish.
Cited by
-
Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid.Nutrients. 2023 Jan 1;15(1):224. doi: 10.3390/nu15010224. Nutrients. 2023. PMID: 36615882 Free PMC article. Review.
-
Potency of butylated hydroxytoluene and optimized black pepper extract as additives on quality characteristics of stored (4 °C) pork on various days.Food Sci Biotechnol. 2024 May 2;33(14):3367-3377. doi: 10.1007/s10068-024-01567-3. eCollection 2024 Nov. Food Sci Biotechnol. 2024. PMID: 39328218 Free PMC article.
-
Phytogenic Water Additives Improve Broiler Growth Performance via Modulation of Intermediary Metabolism-Related Signaling Pathways.Animals (Basel). 2021 Mar 9;11(3):750. doi: 10.3390/ani11030750. Animals (Basel). 2021. PMID: 33803312 Free PMC article.
-
Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer.Cancers (Basel). 2023 Nov 14;15(22):5407. doi: 10.3390/cancers15225407. Cancers (Basel). 2023. PMID: 38001668 Free PMC article.
-
Molecular Mechanisms Underlying the Elevated Expression of a Potentially Type 2 Diabetes Mellitus Associated SCD1 Variant.Int J Mol Sci. 2022 Jun 2;23(11):6221. doi: 10.3390/ijms23116221. Int J Mol Sci. 2022. PMID: 35682900 Free PMC article.
References
-
- Das U.N. Gamma-linolenic acid, arachidonic acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition. 1990;6:429–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

