Phospholipase C inhibits apoptosis of porcine primary granulosa cells cultured in vitro
- PMID: 31554511
- PMCID: PMC6761717
- DOI: 10.1186/s13048-019-0567-4
Phospholipase C inhibits apoptosis of porcine primary granulosa cells cultured in vitro
Abstract
Phospholipase C (PLC) can participate in cell proliferation, differentiation and aging. However, whether it has a function in apoptosis in porcine primary granulosa cells is largely uncertain. The objective of this study was to examine the effects of PLC on apoptosis of porcine primary granulosa cells cultured in vitro. The mRNA expression of BAK, BAX and CASP3, were upregulated in the cells treated with U73122 (the PLC inhibitor). The abundance of BCL2 mRNA, was upregulated, while BAX and CASP3 mRNA expression was decreased after treatment with m-3M3FBS (the PLC activator). Both the early and late apoptosis rate were maximized with 0.5 μM U73122 for 4 h. The rate of early apoptosis was the highest at 4 h and the rate of late apoptosis was the highest at 12 h in the m-3M3FBS group. The protein abundance of PLCβ1, protein kinase C β (PKCβ), calmodulin-dependent protein kinaseII α (CAMKIIα) and calcineurinA (CalnA) were decreased by U73122, and CAMKIIα protein abundance was increased by m-3M3FBS. The mRNA expression of several downstream genes (CDC42, NFATc1, and NFκB) was upregulated by PLC. Our results demonstrated that apoptosis can be inhibited by altering PLC signaling in porcine primary granulosa cells cultured in vitro, and several calcium-sensitive targets and several downstream genes might take part in the processes.
Keywords: Apoptosis; Granulosa cells; Phospholipase C; Porcine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

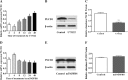
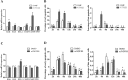
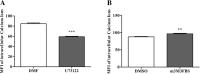
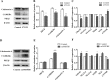
Similar articles
-
Phospholipase C inhibits apoptosis of porcine oocytes cultured in vitro.J Cell Biochem. 2020 Jul;121(7):3547-3559. doi: 10.1002/jcb.29636. Epub 2020 Jan 3. J Cell Biochem. 2020. PMID: 31898356
-
The effects of phospholipase C on oestradiol and progesterone secretion in porcine granulosa cells cultured in vitro.Reprod Domest Anim. 2019 Sep;54(9):1236-1243. doi: 10.1111/rda.13517. Epub 2019 Aug 6. Reprod Domest Anim. 2019. PMID: 31319005
-
The novel phospholipase C activator, m-3M3FBS, induces apoptosis in tumor cells through caspase activation, down-regulation of XIAP and intracellular calcium signaling.Apoptosis. 2008 Jan;13(1):133-45. doi: 10.1007/s10495-007-0159-4. Apoptosis. 2008. PMID: 18060503
-
GnRH-induced calcium mobilisation and inositol phosphate production in immature and mature rat ovarian granulosa cells.J Endocrinol. 1996 Jun;149(3):449-56. doi: 10.1677/joe.0.1490449. J Endocrinol. 1996. PMID: 8691103
-
Let-7g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary.Int J Biochem Cell Biol. 2015 Nov;68:148-57. doi: 10.1016/j.biocel.2015.08.011. Epub 2015 Aug 20. Int J Biochem Cell Biol. 2015. PMID: 26299328
Cited by
-
Impaired degradation of PLCG1 by chaperone-mediated autophagy promotes cellular senescence and intervertebral disc degeneration.Autophagy. 2025 Feb;21(2):352-373. doi: 10.1080/15548627.2024.2395797. Epub 2024 Sep 10. Autophagy. 2025. PMID: 39212196 Free PMC article.
-
Knockdown of CLAUDIN-6 Inhibited Apoptosis and Induced Proliferation of Bovine Cumulus Cells.Int J Mol Sci. 2022 Oct 30;23(21):13222. doi: 10.3390/ijms232113222. Int J Mol Sci. 2022. PMID: 36362009 Free PMC article.
-
Simulated Microgravity Induces the Proliferative Inhibition and Morphological Changes in Porcine Granulosa Cells.Curr Issues Mol Biol. 2021 Dec 10;43(3):2210-2219. doi: 10.3390/cimb43030155. Curr Issues Mol Biol. 2021. PMID: 34940129 Free PMC article.
-
Long non-coding RNA Loc105611671 promotes the proliferation of ovarian granulosa cells and steroid hormone production upregulation of CDC42.Front Vet Sci. 2024 Mar 4;11:1366759. doi: 10.3389/fvets.2024.1366759. eCollection 2024. Front Vet Sci. 2024. PMID: 38500606 Free PMC article.
-
Isorhamnetin protects zearalenone-induced damage via the PI3K/Akt signaling pathway in porcine ovarian granulosa cells.Anim Nutr. 2022 Aug 8;11:381-390. doi: 10.1016/j.aninu.2022.06.019. eCollection 2022 Dec. Anim Nutr. 2022. PMID: 36329687 Free PMC article.
References
-
- D Guthrie H, M Garrett W. Apoptosis during folliculogenesis in pigs. Reprod Suppl. 2001;58:17–29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

