Epigenetic control of transcriptional regulation in pluripotency and early differentiation
- PMID: 31554624
- PMCID: PMC6803368
- DOI: 10.1242/dev.164772
Epigenetic control of transcriptional regulation in pluripotency and early differentiation
Abstract
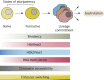
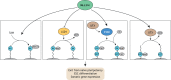
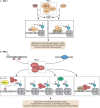
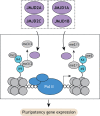
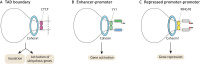
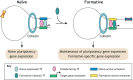
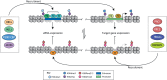
Pluripotent stem cells give rise to all cells of the adult organism, making them an invaluable tool in regenerative medicine. In response to differentiation cues, they can activate markedly distinct lineage-specific gene networks while turning off or rewiring pluripotency networks. Recent innovations in chromatin and nuclear structure analyses combined with classical genetics have led to novel insights into the transcriptional and epigenetic mechanisms underlying these networks. Here, we review these findings in relation to their impact on the maintenance of and exit from pluripotency and highlight the many factors that drive these processes, including histone modifying enzymes, DNA methylation and demethylation, nucleosome remodeling complexes and transcription factor-mediated enhancer switching.
Keywords: Embryonic stem cells; Epiblast-like cells; Epigenetics; Mouse; Pluripotency; Transcriptional regulation.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Blackledge N. P., Farcas A. M., Kondo T., King H. W., McGouran J. F., Hanssen L. L. P., Ito S., Cooper S., Kondo K., Koseki Y. et al. (2014). Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445-1459. 10.1016/j.cell.2014.05.004 - DOI - PMC - PubMed
-
- Bornelöv S., Reynolds N., Xenophontos M., Gharbi S., Johnstone E., Floyd R., Ralser M., Signolet J., Loos R., Dietmann S. et al. (2018). The nucleosome remodeling and deacetylation complex modulates chromatin structure at sites of active transcription to fine-tune gene expression. Mol. Cell 71, 56-72.e4. 10.1016/j.molcel.2018.06.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

